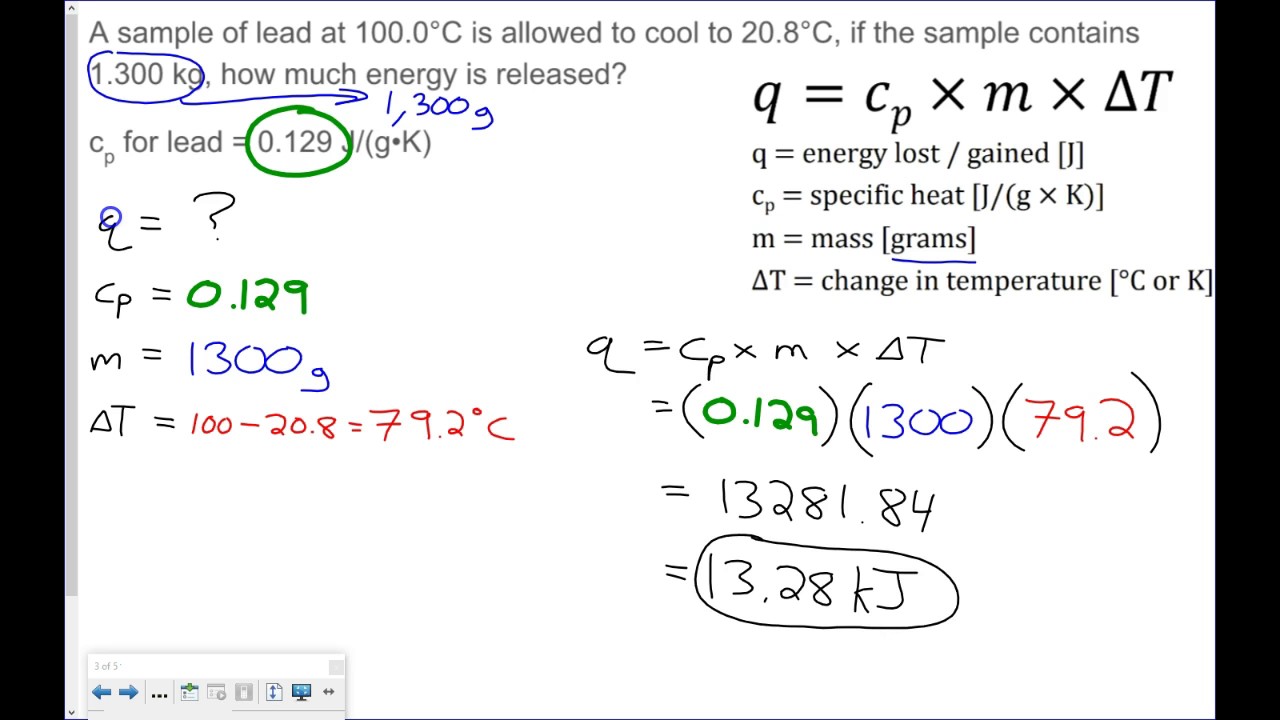
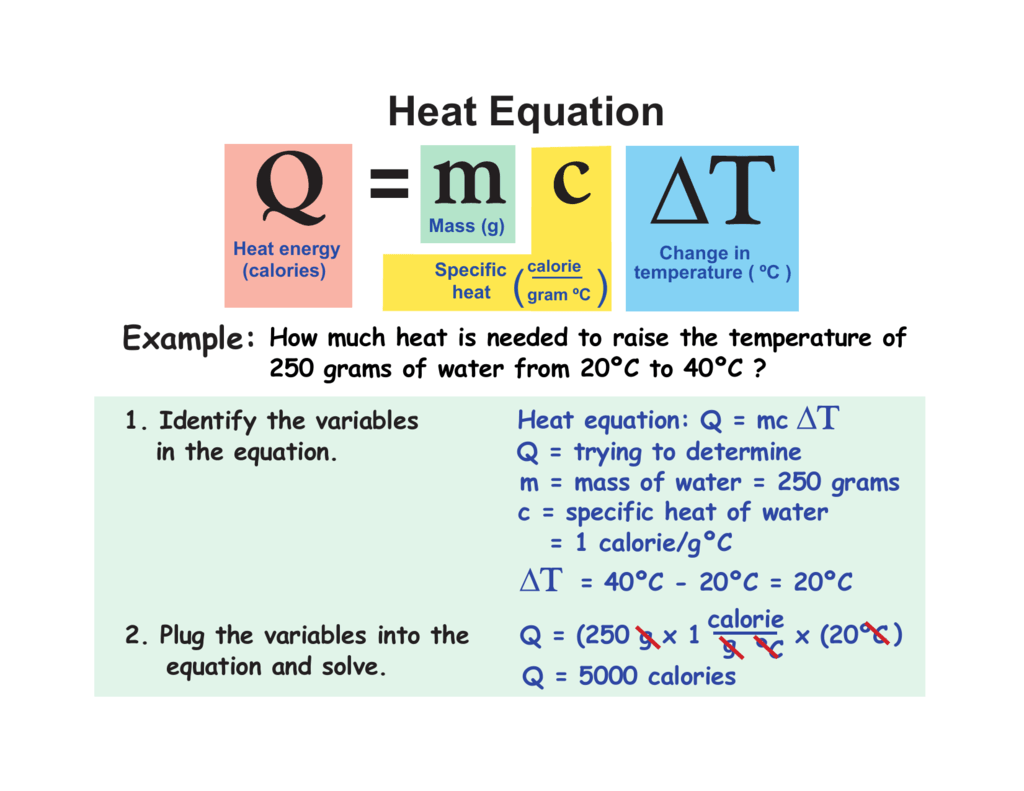
Calculations Involving Specific Heat Worksheet
Calculations Involving Specific Heat Worksheet - For q= m c δ t : Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Calculate the specific heat capacity of iron. Identify each variables by name & the units associated with it. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Identify each variable by name & the units associated with it 2. How much heat did this. For q = m ⋅ c ⋅ δt :
Calculate the specific heat capacity of iron. Identify each variable by name & the units associated with it 2. For q= m c δ t : Identify each variables by name & the units associated with it. How much heat did this. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. For q = m ⋅ c ⋅ δt : Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to.
Identify each variable by name & the units associated with it 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Identify each variables by name & the units associated with it. How much heat did this. For q= m c δ t : Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Calculate the specific heat capacity of iron. For q = m ⋅ c ⋅ δt :
Specific heat calculationsconverted Part 1 Calculating
Identify each variable by name & the units associated with it 2. Calculate the specific heat capacity of iron. For q= m c δ t : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How many joules of heat are needed to raise the temperature of 10.0 g of.
Worksheet Calculations Involving Specific Heat
Identify each variable by name & the units associated with it 2. Identify each variables by name & the units associated with it. For q = m ⋅ c ⋅ δt : Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to.
Calculating Specific Heat Worksheet
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How much heat did this. Calculate the specific heat capacity of iron. Identify each variable by name & the units associated with it 2. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc.
Calculations involving Specific Heat Worksheet Exercises
Identify each variable by name & the units associated with it 2. For q= m c δ t : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. For q = m ⋅ c ⋅ δt : Specific heat and heat capacity worksheet 1 the temperature of 335 g of.
Calculating Specific Heat Extra Practice Worksheet
Calculate the specific heat capacity of iron. For q = m ⋅ c ⋅ δt : For q= m c δ t : Calculate the specific heat capacity of iron. Identify each variables by name & the units associated with it.
Worksheet Calculations involving Specific Heat
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How much heat.
10++ Specific Heat Worksheet Worksheets Decoomo
Calculate the specific heat capacity of iron. Calculate the specific heat capacity of iron. How much heat did this. Identify each variables by name & the units associated with it. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc.
Worksheet Calculation Involving Specific Heat
For q = m ⋅ c ⋅ δt : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. For q= m c δ t : Calculate the specific heat capacity of iron. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to.
Worksheet Calculation Involving Specific Heat
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. For q = m ⋅ c ⋅ δt : Identify each variables by name &.
Identify Each Variable By Name & The Units Associated With It 2.
For q= m c δ t : For q = m ⋅ c ⋅ δt : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc.
How Much Heat Did This.
Calculate the specific heat capacity of iron. Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Identify each variables by name & the units associated with it.