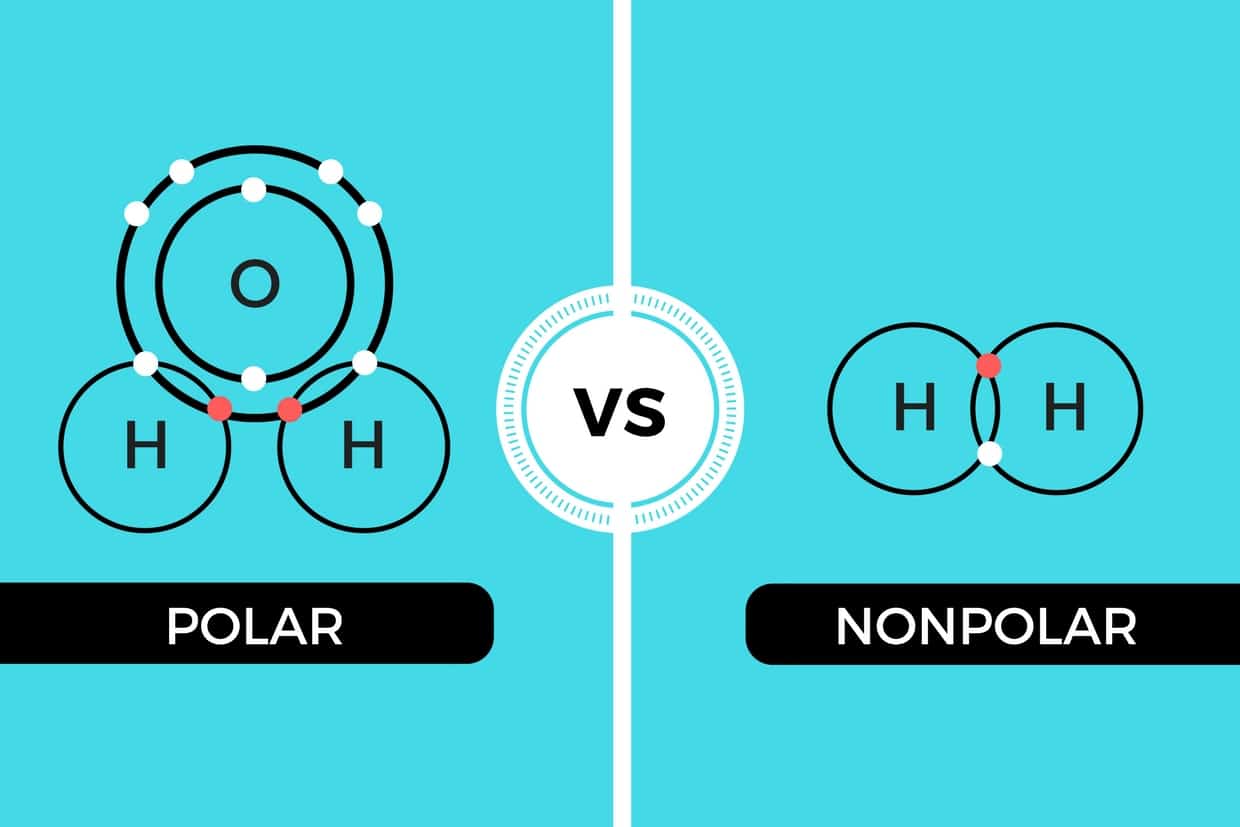
Can Carbon Form Polar And Nonpolar Bonds
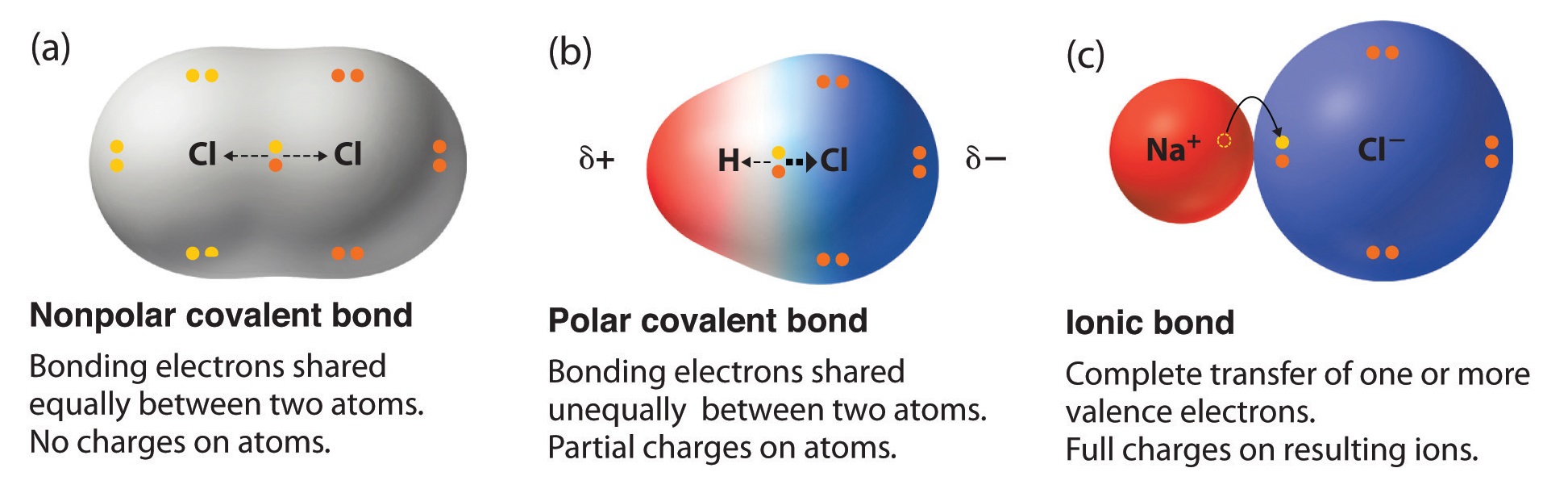
Can Carbon Form Polar And Nonpolar Bonds - Single or multiple bonds between carbon atoms are nonpolar. In fact, carbon is known to form polar bonds with a wide variety. Carbon bonds are stable across a broad range of temperatures. Carbon can form both polar and nonpolar bonds. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Is a carbon carbon bond polar or nonpolar? Can carbon form polar bonds? Yes, carbon can form polar bonds.
Single or multiple bonds between carbon atoms are nonpolar. In fact, carbon is known to form polar bonds with a wide variety. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Can carbon form polar bonds? Carbon bonds are stable across a broad range of temperatures. Yes, carbon can form polar bonds. Is a carbon carbon bond polar or nonpolar? Carbon can form both polar and nonpolar bonds.
Is a carbon carbon bond polar or nonpolar? Can carbon form polar bonds? Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. In fact, carbon is known to form polar bonds with a wide variety. Carbon bonds are stable across a broad range of temperatures. Yes, carbon can form polar bonds. Carbon can form both polar and nonpolar bonds. Single or multiple bonds between carbon atoms are nonpolar.
Polar vs. Nonpolar Bonds — Overview & Examples Expii Chemistry
Is a carbon carbon bond polar or nonpolar? Carbon bonds are stable across a broad range of temperatures. In fact, carbon is known to form polar bonds with a wide variety. Yes, carbon can form polar bonds. Can carbon form polar bonds?
Chapter 2. The Chemical Context of Life Introduction to Molecular and
Is a carbon carbon bond polar or nonpolar? Can carbon form polar bonds? Single or multiple bonds between carbon atoms are nonpolar. Yes, carbon can form polar bonds. Carbon bonds are stable across a broad range of temperatures.
De este modo Resignación borde can carbon form polar and nonpolar bonds
Carbon bonds are stable across a broad range of temperatures. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. In fact, carbon is known to form polar bonds with a wide variety. Is a carbon carbon bond polar or nonpolar? Yes, carbon can form polar bonds.
Polar vs. Nonpolar Bonds — Overview & Examples Expii Chemistry
Can carbon form polar bonds? Carbon can form both polar and nonpolar bonds. Single or multiple bonds between carbon atoms are nonpolar. In fact, carbon is known to form polar bonds with a wide variety. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond.
Ms J's Chemistry Class Polar vs NonPolar Covalent Bonds
Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Is a carbon carbon bond polar or nonpolar? Can carbon form polar bonds? Carbon bonds are stable across a broad range of temperatures. Yes, carbon can form polar bonds.
Polar Molecule CHEMISTRY COMMUNITY
Carbon bonds are stable across a broad range of temperatures. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. In fact, carbon is known to form polar bonds with a wide variety. Yes, carbon can form polar bonds. Carbon can form both polar and nonpolar bonds.
Which Describes Bonding Electrons in a Polar Covalent Bond JakekruwBauer
Single or multiple bonds between carbon atoms are nonpolar. Carbon bonds are stable across a broad range of temperatures. Carbon can form both polar and nonpolar bonds. Is a carbon carbon bond polar or nonpolar? In fact, carbon is known to form polar bonds with a wide variety.
Definition and Examples of a Polar Bond
Carbon can form both polar and nonpolar bonds. Is a carbon carbon bond polar or nonpolar? Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Carbon bonds are stable across a broad range of temperatures. In fact, carbon is known to form polar bonds with a wide variety.
2.1 Covalent Bonds Biology LibreTexts
Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Can carbon form polar bonds? Is a carbon carbon bond polar or nonpolar? Carbon bonds are stable across a broad range of temperatures. Single or multiple bonds between carbon atoms are nonpolar.
How To Find The Polarity Of Bonds
Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. Carbon bonds are stable across a broad range of temperatures. Is a carbon carbon bond polar or nonpolar? Single or multiple bonds between carbon atoms are nonpolar. Can carbon form polar bonds?
In Fact, Carbon Is Known To Form Polar Bonds With A Wide Variety.
Single or multiple bonds between carbon atoms are nonpolar. Carbon can form both polar and nonpolar bonds. Yes, carbon can form polar bonds. Is a carbon carbon bond polar or nonpolar?
Carbon Bonds Are Stable Across A Broad Range Of Temperatures.
Can carbon form polar bonds? Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond.

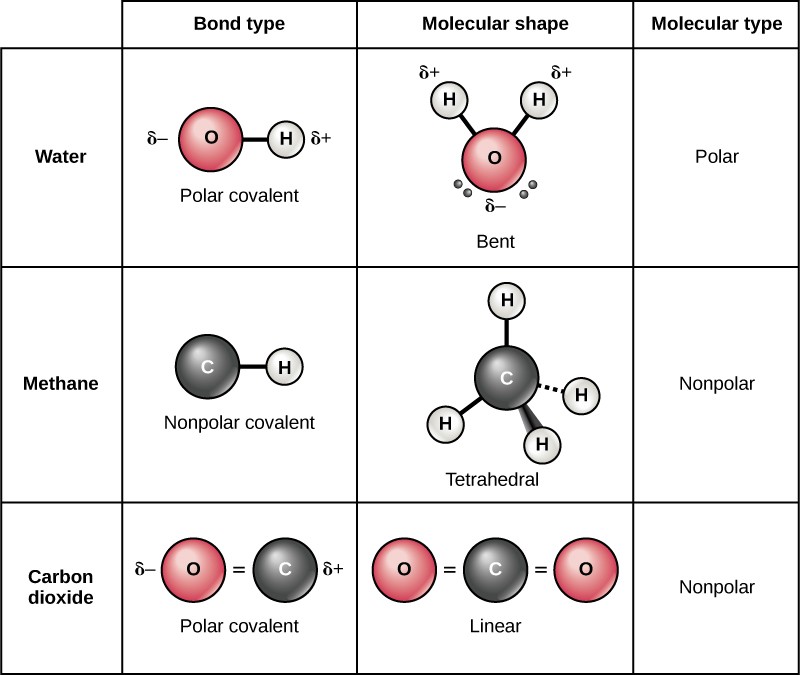


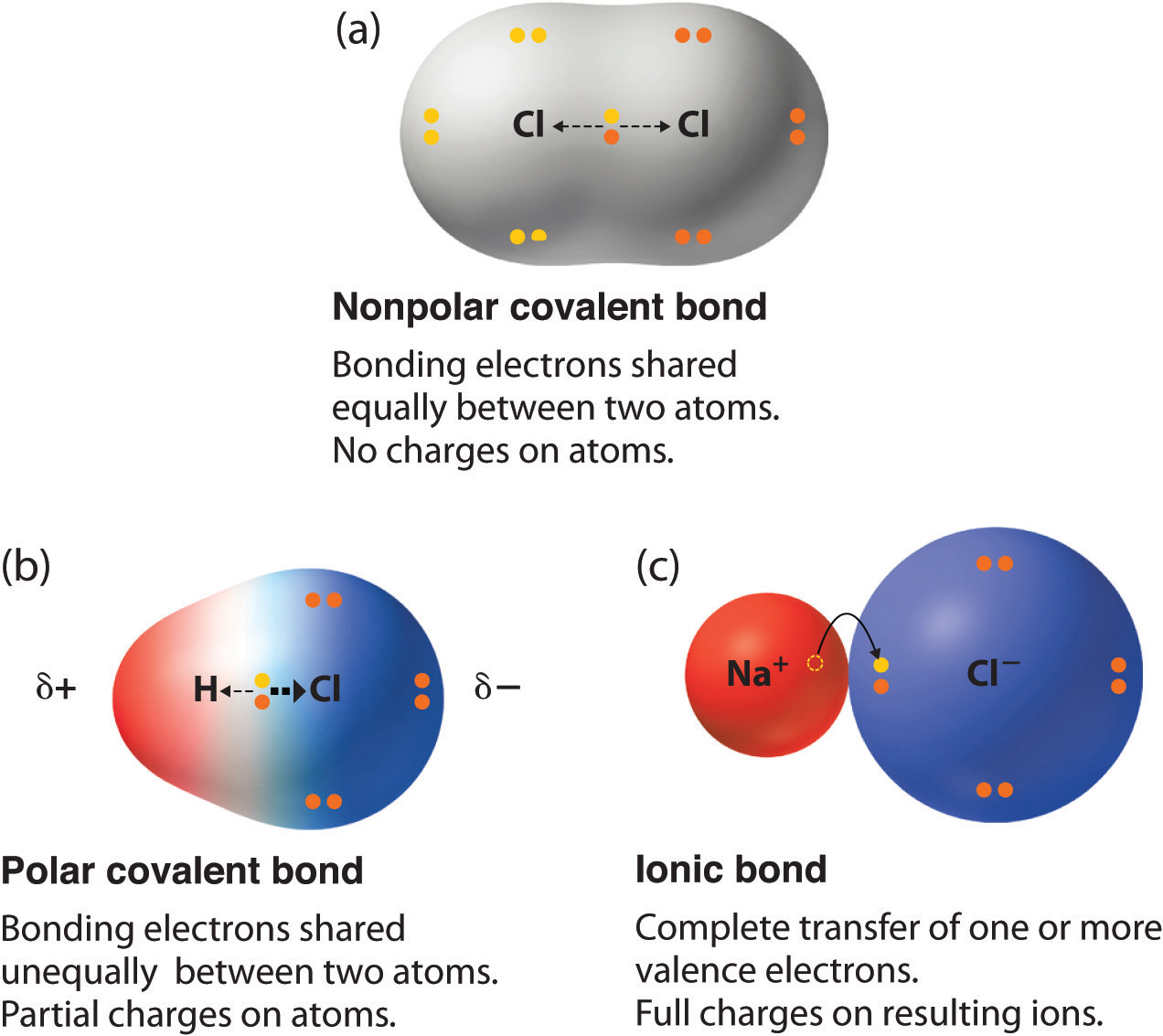
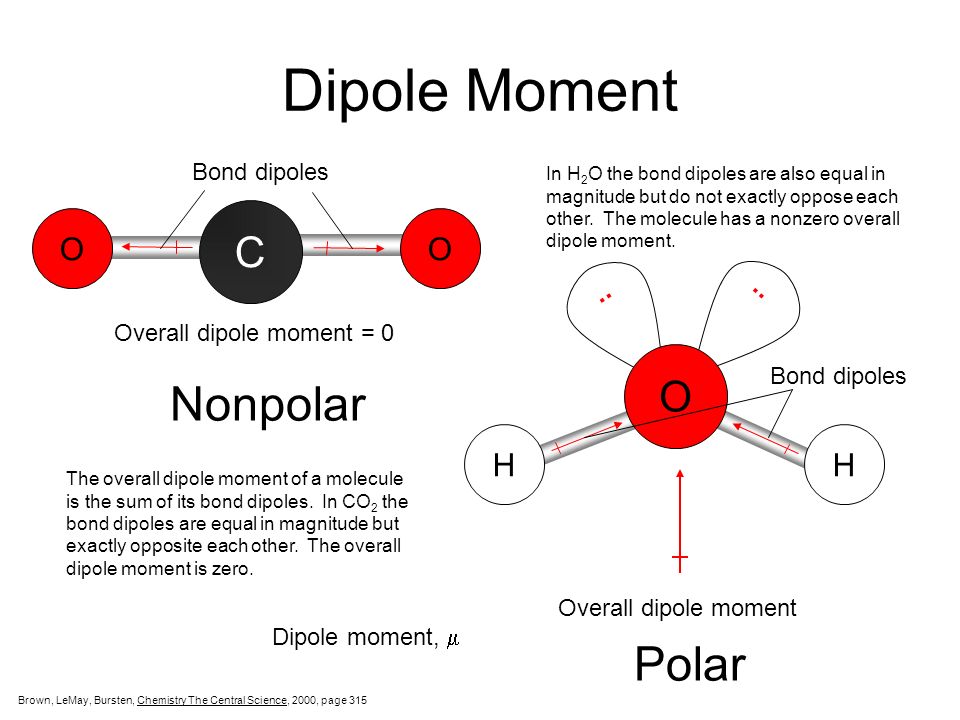
:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)