Emission Spectra And Energy Levels Worksheet Answers
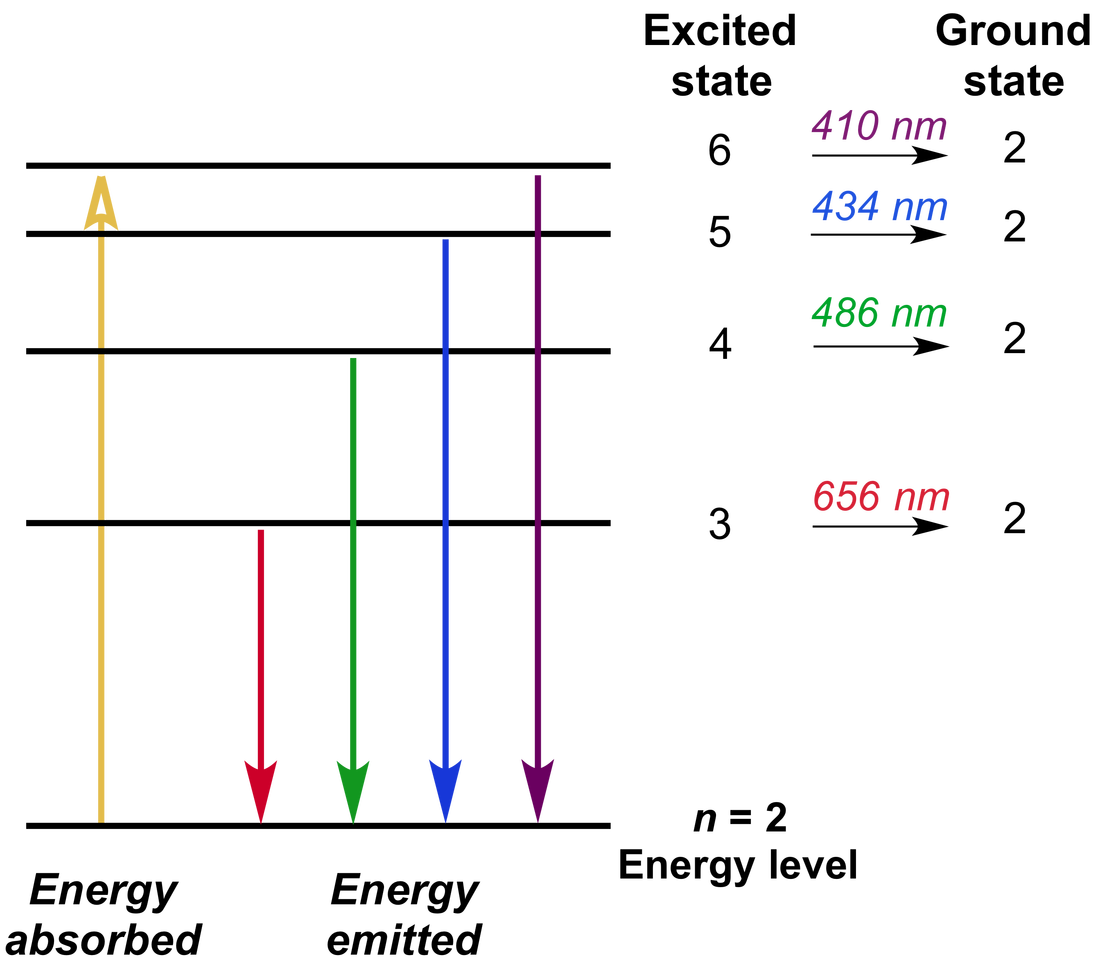
Emission Spectra And Energy Levels Worksheet Answers - The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Emission spectra and energy levels worksheet answers: Breaking away from the traditional compendia of emission lines the database has been compiled using an algorithm which calculated all the. Your solution’s ready to go! Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels. Movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite,. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6.
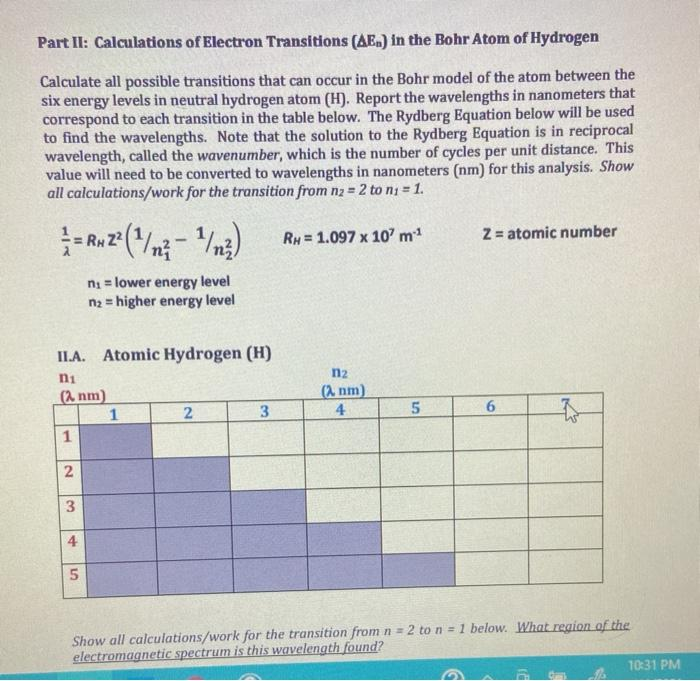
Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. Breaking away from the traditional compendia of emission lines the database has been compiled using an algorithm which calculated all the. Movement of an electron from one discrete energy level to another. Thus, emission spectra are experimental proof that electrons exist in definite,. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Emission spectra and energy levels worksheet answers: Your solution’s ready to go! The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels.
Movement of an electron from one discrete energy level to another. Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. Thus, emission spectra are experimental proof that electrons exist in definite,. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Your solution’s ready to go! Breaking away from the traditional compendia of emission lines the database has been compiled using an algorithm which calculated all the. The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels. Emission spectra and energy levels worksheet answers:
Chemistry Electron Emission Spectrum
Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels. Your solution’s ready to go! Emission spectra and energy levels worksheet answers: The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Breaking away from the traditional.
Emission Spectra And Energy Levels Worksheets Answers
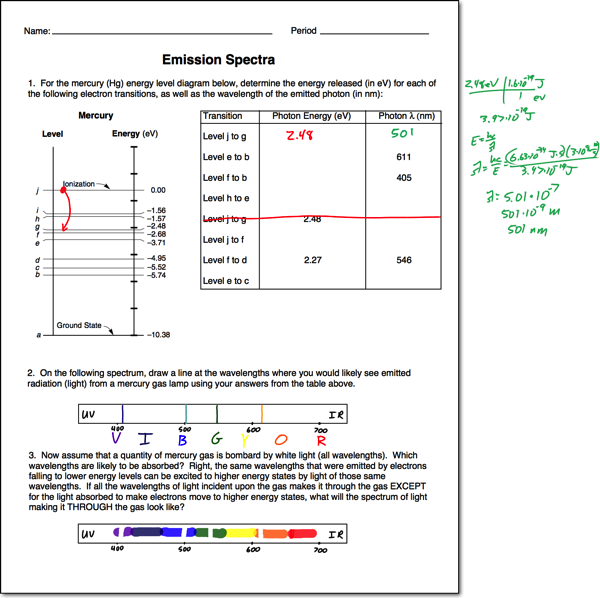
The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. Thus, emission spectra are experimental proof that electrons exist in definite,. Electrons absorb energy from various.
the diagram shows how light is reflected in an image
Movement of an electron from one discrete energy level to another. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Breaking away from the traditional compendia of emission lines the database has been compiled using an algorithm which calculated all the. Your solution’s ready to go! Emission spectra.
Capcd Emission Factor Emission Worksheet
Movement of an electron from one discrete energy level to another. The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Emission spectra and energy levels worksheet answers: Thus, emission spectra are experimental proof that electrons exist in definite,. Your solution’s ready to go!
EMISSION SPECTRA AND ENERGY LEVELS
Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. Your solution’s ready to go! The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Electrons absorb energy from various sources, such as heat, light, or.
Emission Spectra And Energy Levels Worksheet Answers Printable Word
Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels. The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n.
Atomic Emission Spectrum Worksheet
Your solution’s ready to go! Thus, emission spectra are experimental proof that electrons exist in definite,. The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6..
Emission Spectra And Energy Levels Worksheet Answers Doc, 60 OFF
Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. Thus, emission spectra are experimental proof that electrons exist in definite,. The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Breaking away from the traditional.
Emission Spectra And Energy Levels Worksheet Ivuyteq
Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. Your solution’s ready to go! Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels. The emission spectrum corresponds to the transitions from the energy levels of state s.
Emission Spectra and Energy Levels Worksheet Answers airSlate SignNow
Your solution’s ready to go! The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Emission spectra and energy levels worksheet answers: Electrons absorb energy from.
Thus, Emission Spectra Are Experimental Proof That Electrons Exist In Definite,.
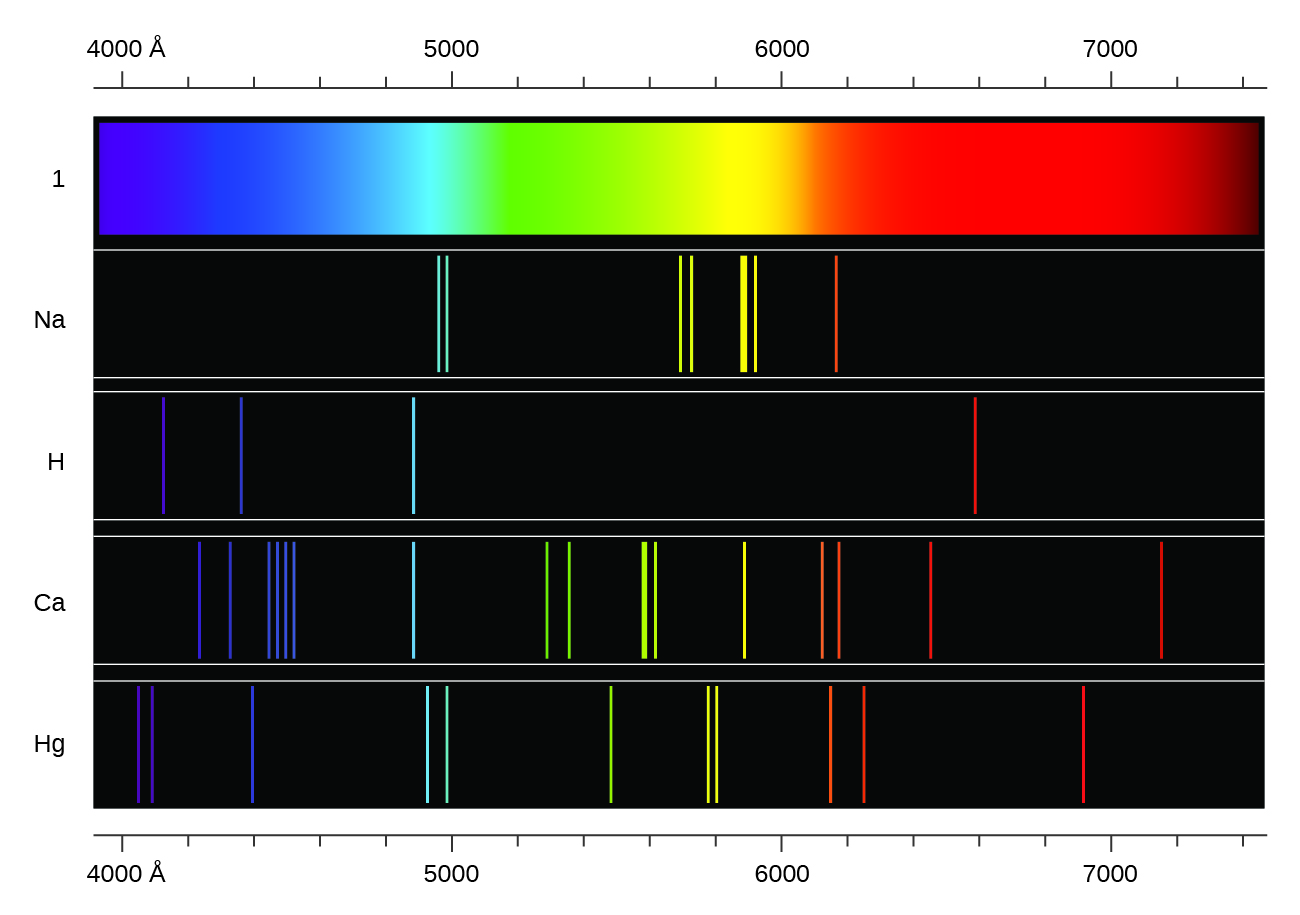
The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Electrons absorb energy from various sources, such as heat, light, or electricity, and the electrons move from lower energy levels. Movement of an electron from one discrete energy level to another. Your solution’s ready to go!
Emission Spectra And Energy Levels Worksheet Answers:
Using the equation below derived from the rydberg equation, calculate the energy levels (e.) energy from n = 1 to 6. The emission spectrum corresponds to the transitions from the energy levels of state s 1 to the energy levels of state s 0. Breaking away from the traditional compendia of emission lines the database has been compiled using an algorithm which calculated all the.