Formdo Noble Gases Form Ions
Formdo Noble Gases Form Ions - Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. The noble gases are very unreactive. Helium, neon, argon, krypton and xenon are in. Flame tests identify alkali metal ions in compounds.
Flame tests identify alkali metal ions in compounds. Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. The noble gases are very unreactive. Helium, neon, argon, krypton and xenon are in.
Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Helium, neon, argon, krypton and xenon are in. The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds.
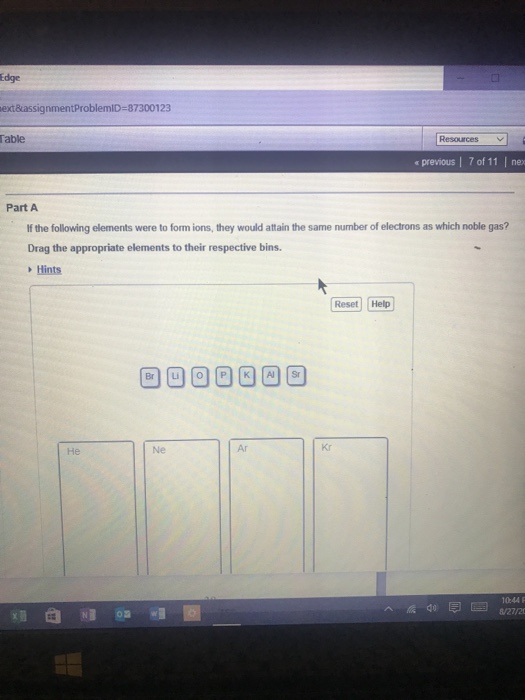
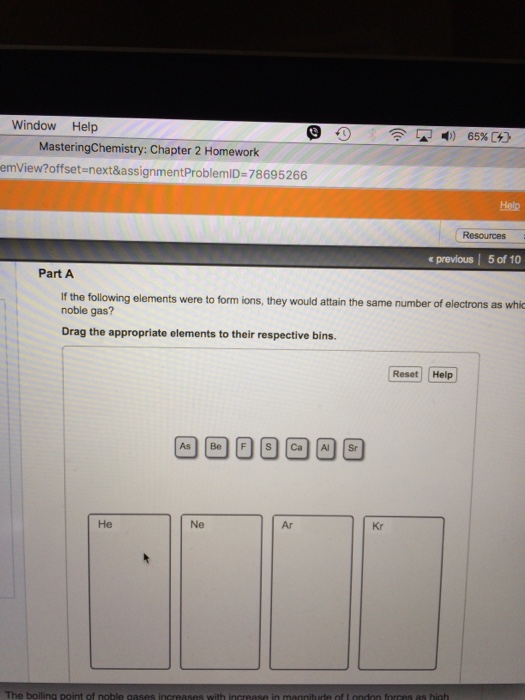
Solved If the following elements were to form ions, they
The noble gases are very unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Their electron configuration does not easily. Flame tests identify alkali metal ions in compounds. Noble gases have a full outer electron shell, making them stable and unreactive.
Why Don't Noble Gases Bond? Lesson
Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive. Flame tests identify alkali metal ions in compounds. Helium, neon, argon, krypton and xenon are in. The noble gases are very unreactive.
Noble Gas Chemical Compounds
The noble gases are very unreactive. Noble gases have a full outer electron shell, making them stable and unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Helium, neon, argon, krypton and xenon are in. Flame tests identify alkali metal ions in compounds.
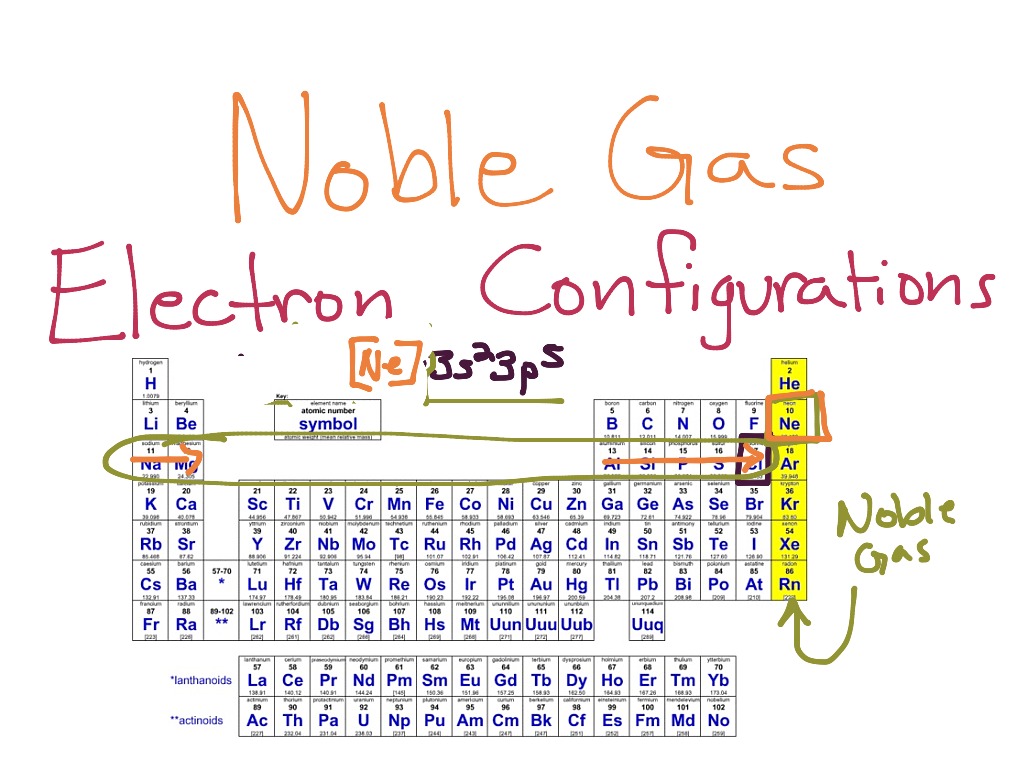
Noble Gases Electron Configuration
Noble gases have a full outer electron shell, making them stable and unreactive. Flame tests identify alkali metal ions in compounds. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Their electron configuration does not easily. The noble gases are very unreactive.
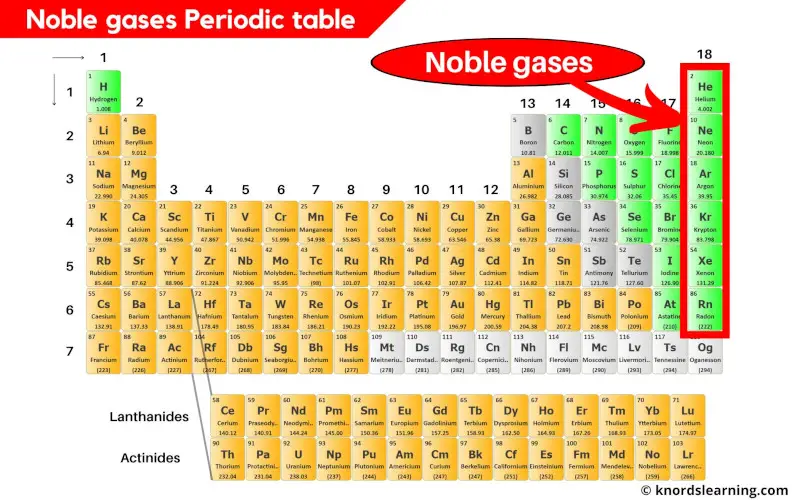
Noble Gases Periodic Table (With Images)
Noble gases have a full outer electron shell, making them stable and unreactive. Helium, neon, argon, krypton and xenon are in. The noble gases are very unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Flame tests identify alkali metal ions in compounds.
Solved If the following elements were to form ions, they
The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Helium, neon, argon, krypton and xenon are in. The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds. Noble gases have a full outer electron shell, making them stable and unreactive.
Noble Gas Chemical Compounds
The noble gases are very unreactive. Helium, neon, argon, krypton and xenon are in. Their electron configuration does not easily. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Noble gases have a full outer electron shell, making them stable and unreactive.
Noble Gases Electron Configuration
Helium, neon, argon, krypton and xenon are in. Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. The noble gases are very unreactive.
coordination compounds Why do full shell ions such as chloride form
Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive. Helium, neon, argon, krypton and xenon are in. Flame tests identify alkali metal ions in compounds. The noble gases are very unreactive.
SOLVEDThe noble gases are sometimes called "inert gases." Why? Why do
Helium, neon, argon, krypton and xenon are in. Noble gases have a full outer electron shell, making them stable and unreactive. The noble gases are very unreactive. Flame tests identify alkali metal ions in compounds. The sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level.
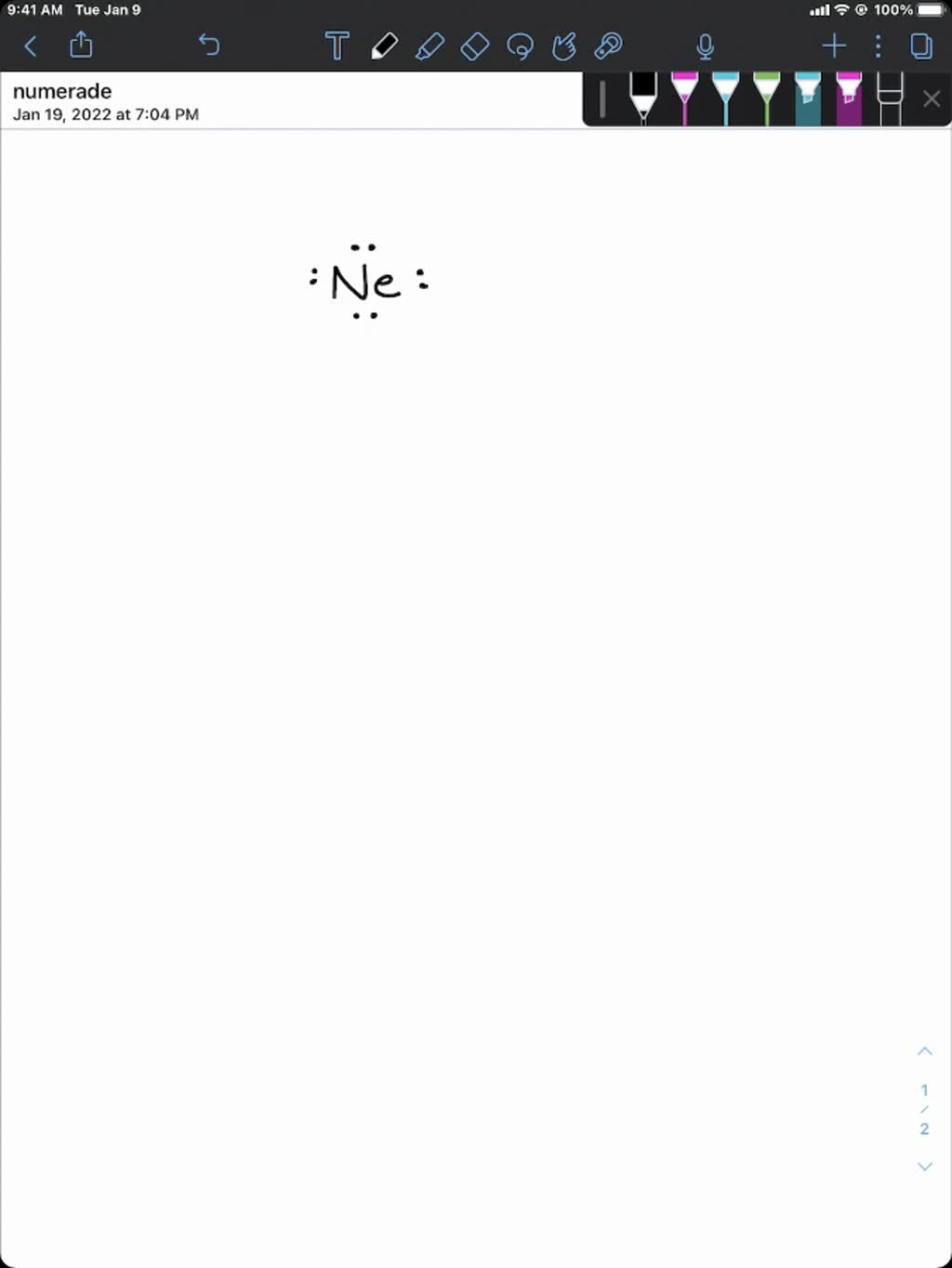
Helium, Neon, Argon, Krypton And Xenon Are In.
Flame tests identify alkali metal ions in compounds. The noble gases are very unreactive. Their electron configuration does not easily. Noble gases have a full outer electron shell, making them stable and unreactive.

:max_bytes(150000):strip_icc()/Xenonhexafluoride-56a12d265f9b58b7d0bccc78.png)

/Xenonhexafluoride-56a12d265f9b58b7d0bccc78.png)