Gamp 5 Full Form
Gamp 5 Full Form - Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Good automated manufacturing practice, founded in 1991. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for.
Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for.
Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. What are gamp® 5 categories, requirements, and. Good automated manufacturing practice, founded in 1991. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems.
GAMP5 GUIDELINES PDF
What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for..
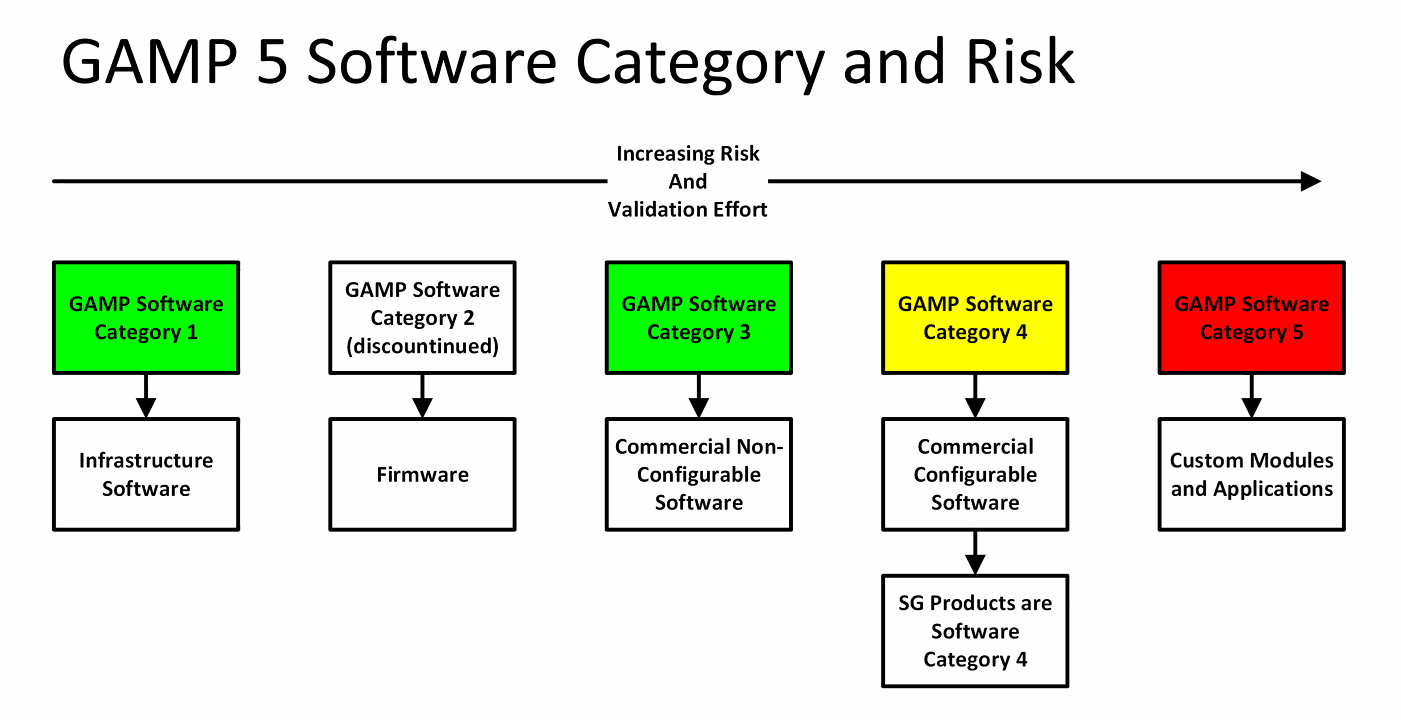
V5 GAMP 5 Software Category & Risk SG Systems Global
Good automated manufacturing practice, founded in 1991. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. What are gamp® 5 categories, requirements, and. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Gamp 5 provides a structured approach to the validation of computerized systems used.
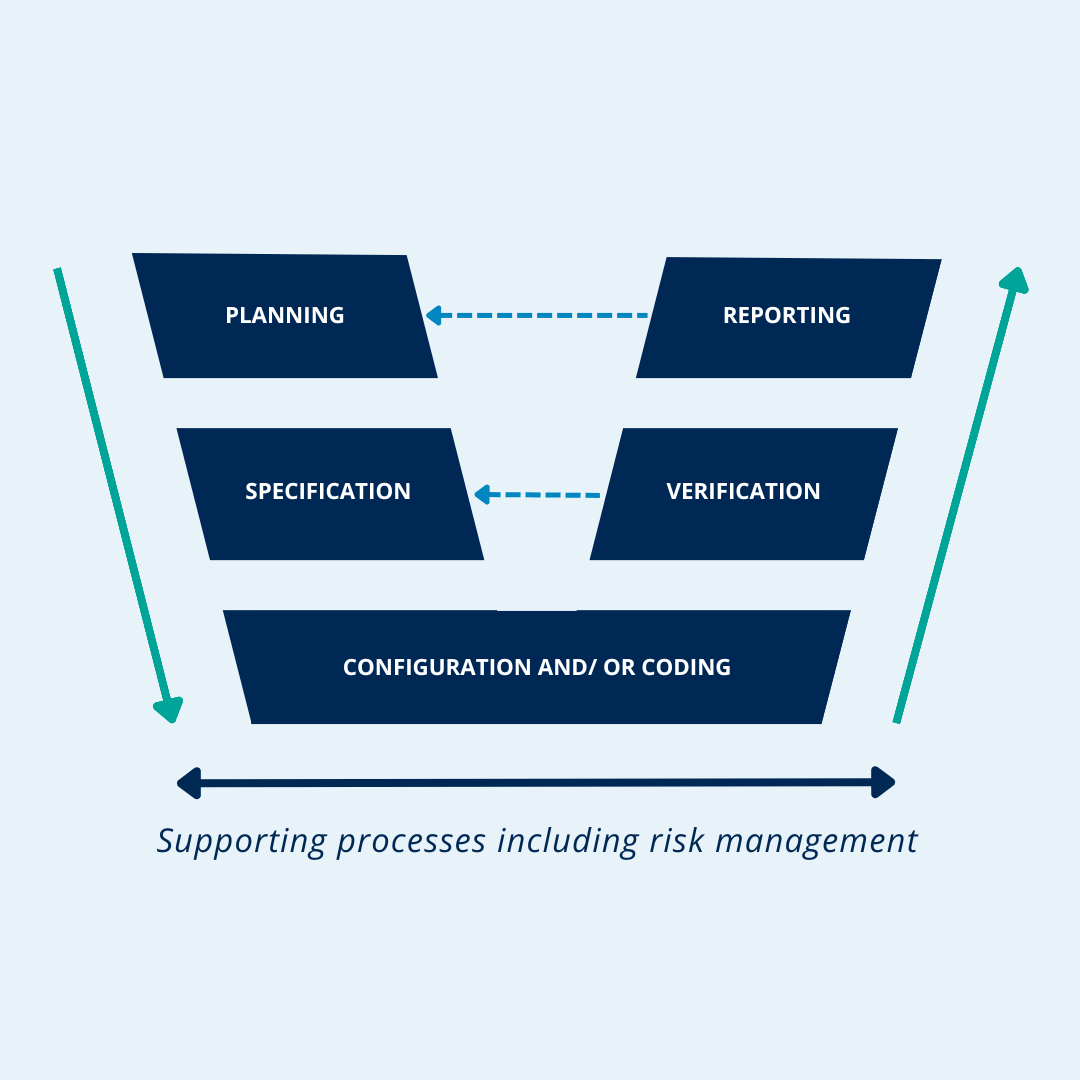
What is the GAMP 5 Vmodel in CSV? QbD Group
Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Good automated manufacturing practice, founded in.
What is the GAMP 5 Vmodel in CSV? QbD Group
Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. What are gamp® 5 categories, requirements, and. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Good automated manufacturing practice, founded in 1991. The gamp® 5 guidelines are.
GAMP 5 Uncovered What You Need to Know Scilife
Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5 provides a structured approach to the validation of computerized systems.
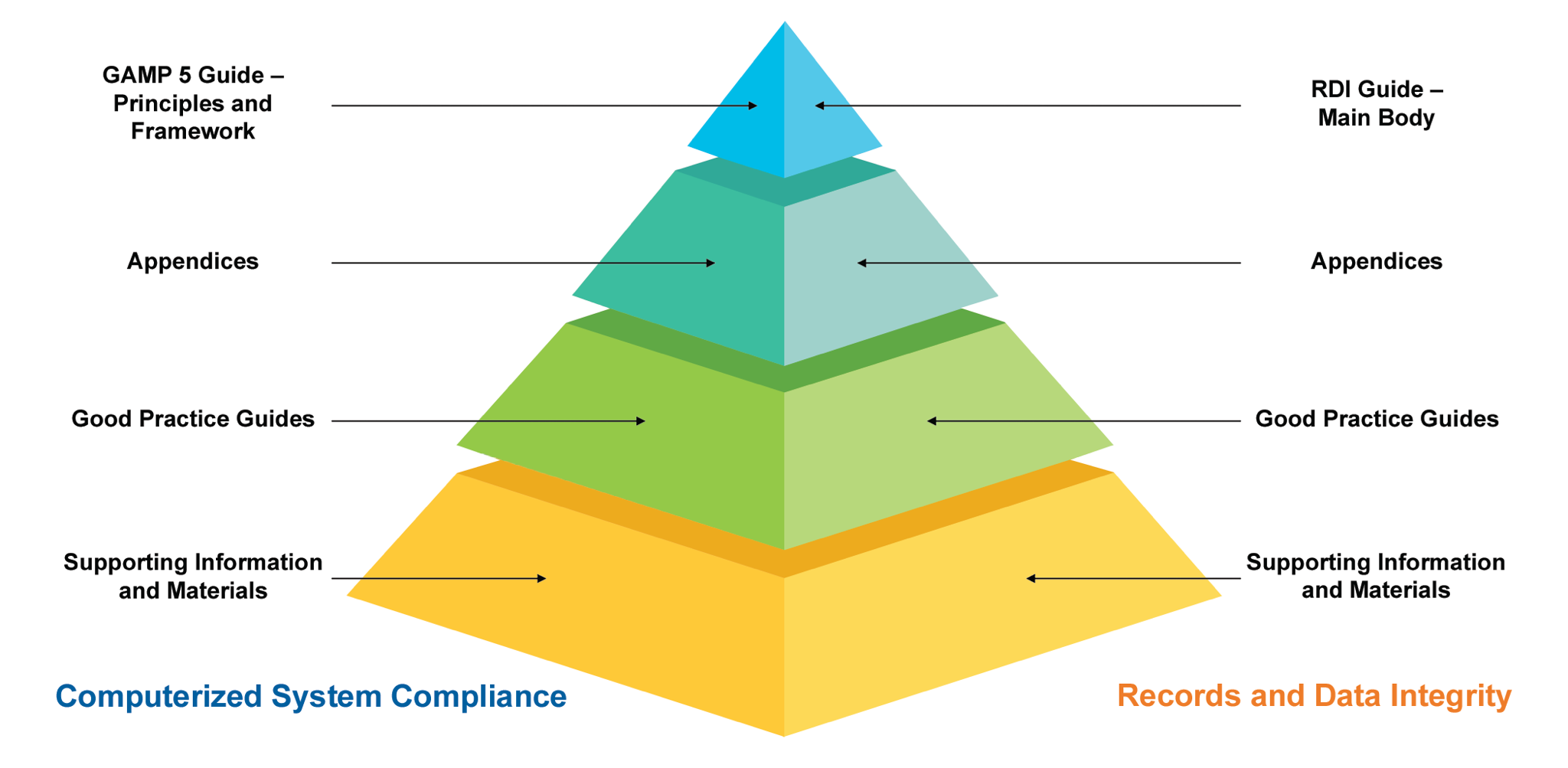
GAMP 5 and GAMP 5 2nd Edition What are the main differences? Scilife
The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. What are gamp® 5 categories, requirements, and. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in.
ISPE GAMP5 second edition What’s new?
Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5, which stands for good automated manufacturing practice, is a set of.
GAMP 5 Uncovered What You Need to Know Scilife
Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. Gamp 5, which stands for good automated manufacturing practice, is a set of guidelines created by the international society for. Gamp 5 provides a structured.
GAMP 5 and GAMP 5 2nd Edition What are the main differences? Scilife
Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the. Good automated manufacturing practice, founded in 1991. What are gamp® 5 categories, requirements, and. The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5 provides a structured approach to the validation of computerized systems.
GAMP 5 and GAMP 5 2nd Edition What are the main differences? Scilife
The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. What are gamp® 5 categories, requirements, and. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in.
Gamp 5, Which Stands For Good Automated Manufacturing Practice, Is A Set Of Guidelines Created By The International Society For.
The gamp® 5 guidelines are essential for ensuring compliant, efficient pharma manufacturing systems. Gamp 5 provides a structured approach to the validation of computerized systems used in the manufacturing and testing of pharmaceutical. Good automated manufacturing practice, founded in 1991. Gamp (good automated manufacturing practice) is a set of guidelines and best practices for the validation of automated systems in the.