Isotopes Are Different Forms Of The Same Element That
Isotopes Are Different Forms Of The Same Element That - 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons.
This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in.
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
What is an Isotope Definition of Isotope
Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in.
DOE Explains...Isotopes Department of Energy
Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount.
Definition and Examples of Isotopes in Chemistry
Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons.
PPT Chapter 4 Atomic Structure PowerPoint Presentation, free download
This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
Isotopes and Ions Seventh Grade Science. ppt download
This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that contain different numbers of neutrons.
PPT Understanding Isotopes and Their Importance in Chemistry
Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
Definition Of Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in.
"Isotopes and their uses"
This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
2 Naturally Occuring Isotopes For Lithium Chemistry International
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons.
Compound Interest ChemistryAdvent IYPT2019 Day 20 A periodic table
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. Isotopes are atoms of the same element that contain different numbers of neutrons.
Isotopes Are Atoms Of The Same Element That Have Different Numbers Of Neutrons And, Therefore, Different Masses.
This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in.
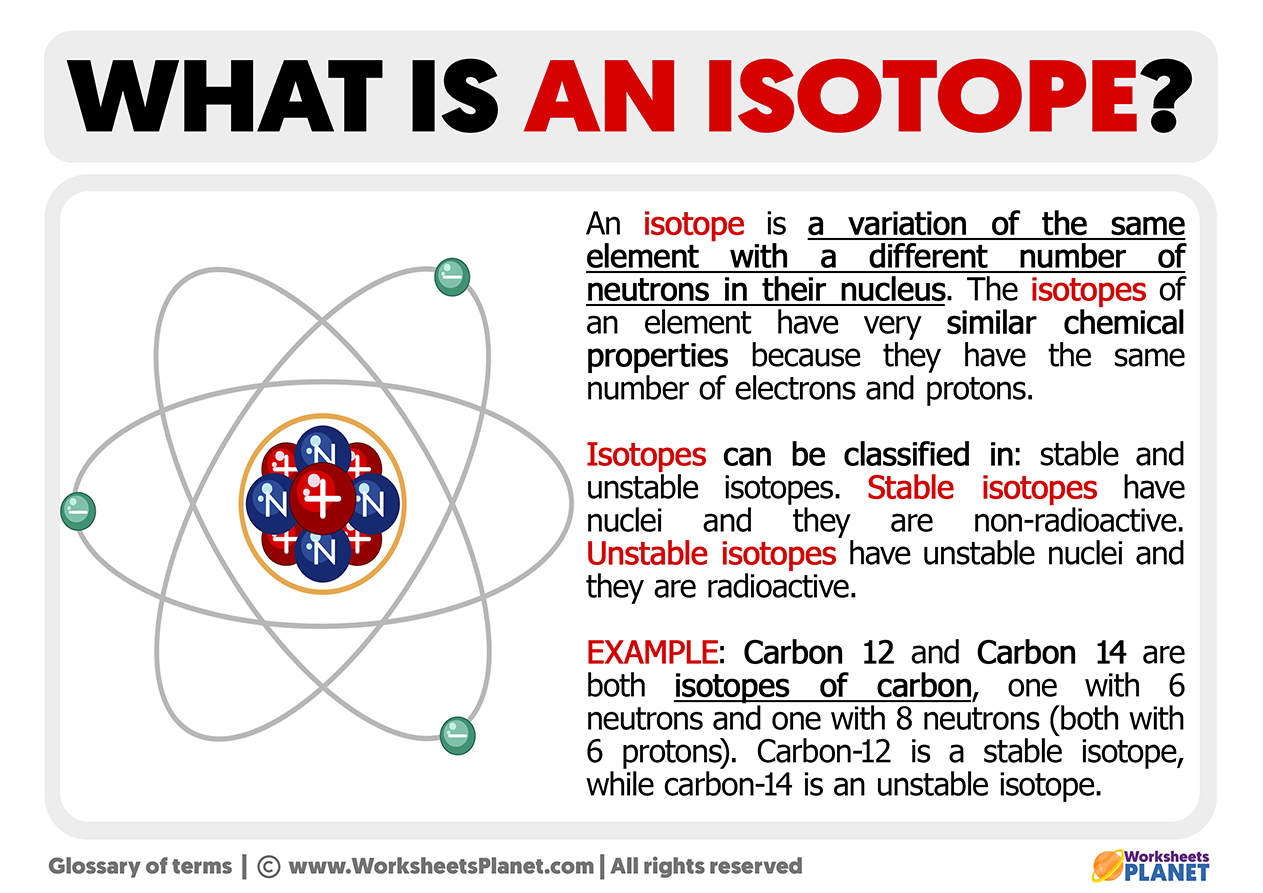
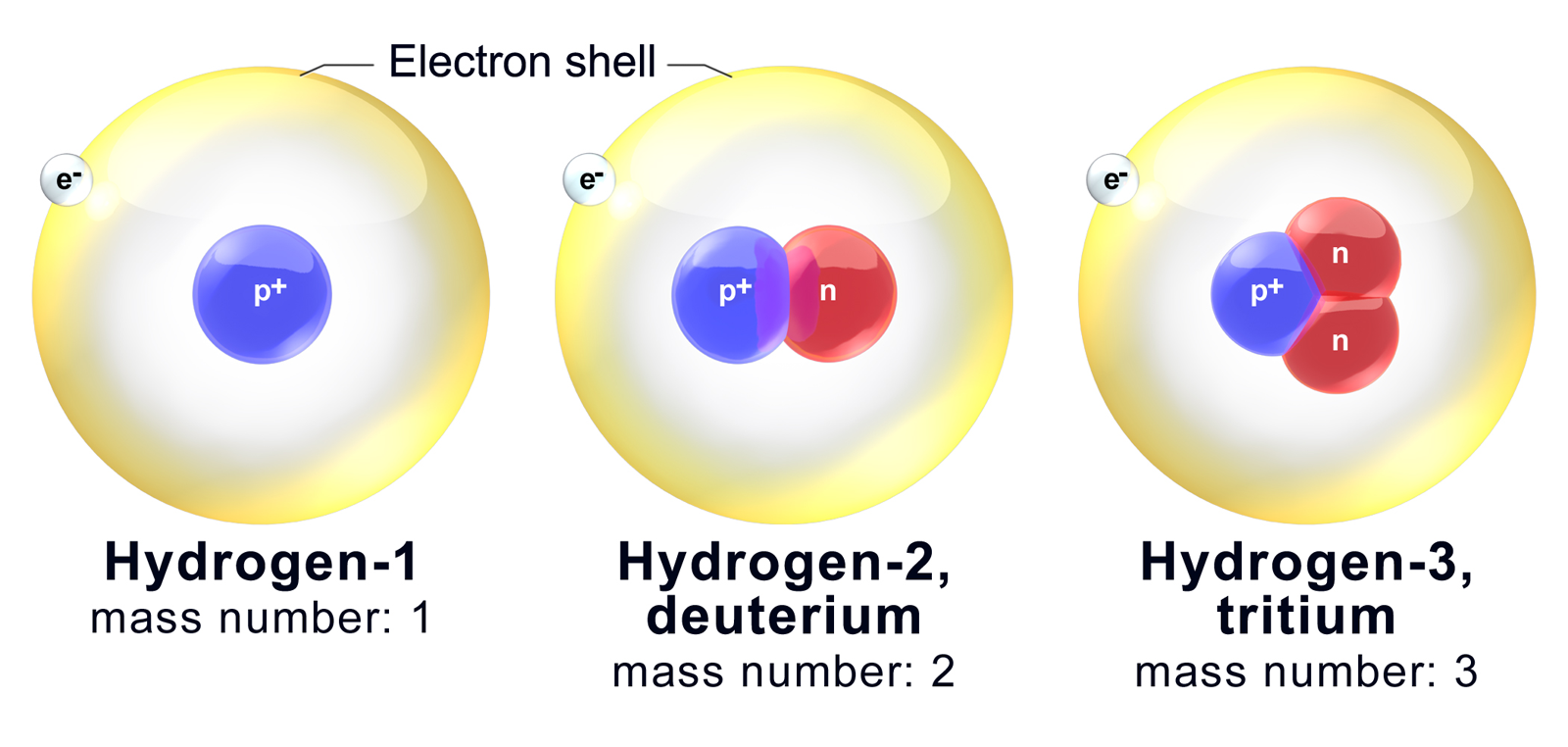
/Isotope-58dd6b415f9b5846830254ae.jpg)
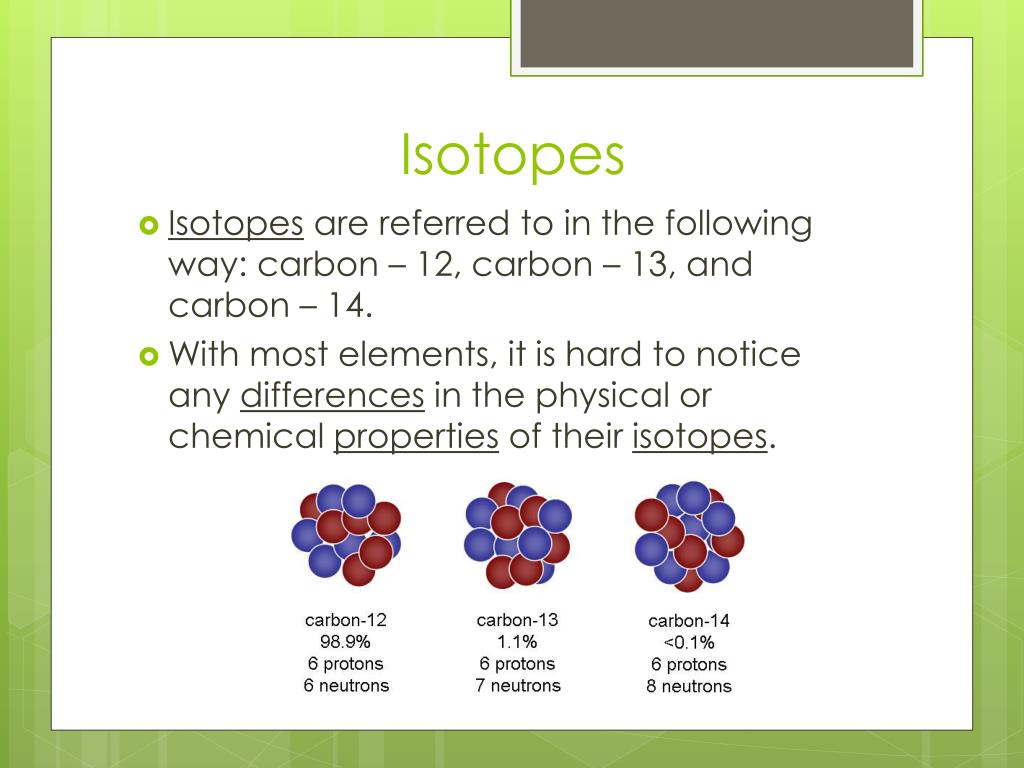
.jpg)




