Magnesium And Oxygen Combine To Form Which Ionic Compound
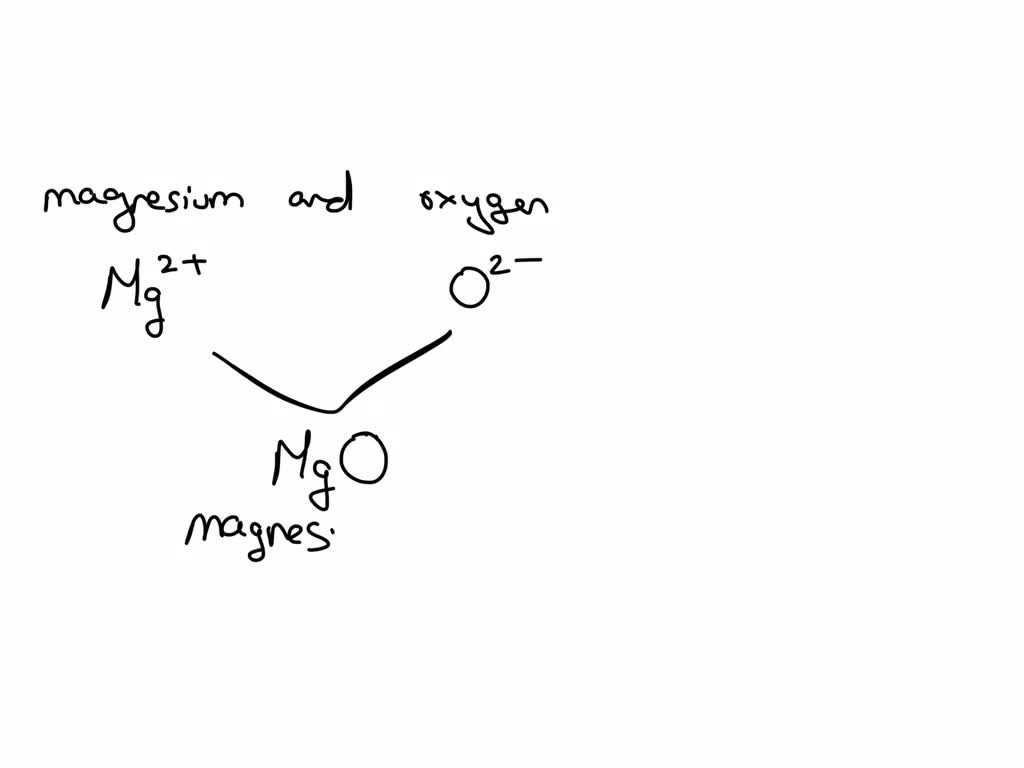
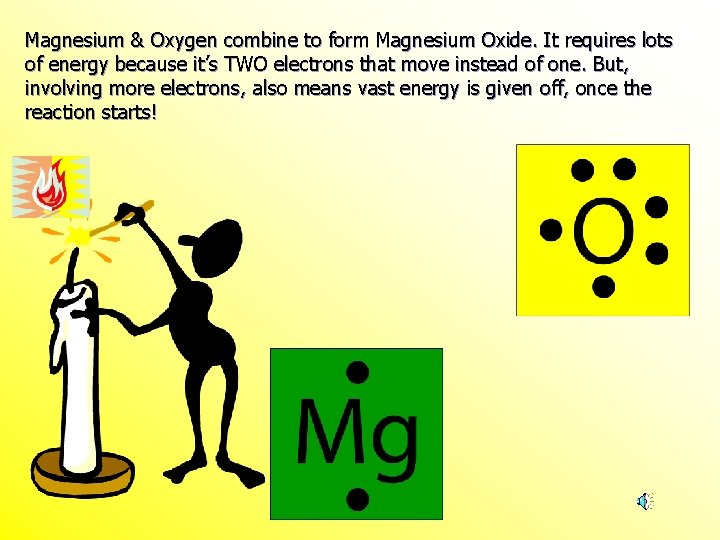
Magnesium And Oxygen Combine To Form Which Ionic Compound - This occurs when magnesium loses. The burning of magnesium metal reacts with oxygen found in the. Magnesium, a metal, gives up two electrons to. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). This is because magnesium (mg) loses 2. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −.
This is because magnesium (mg) loses 2. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. The burning of magnesium metal reacts with oxygen found in the. This occurs when magnesium loses. Magnesium, a metal, gives up two electrons to. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s).
This occurs when magnesium loses. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. Magnesium, a metal, gives up two electrons to. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. This is because magnesium (mg) loses 2. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). The burning of magnesium metal reacts with oxygen found in the. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo.
SOLVED Write net ionic equation for the reaction that occurs when
This occurs when magnesium loses. Magnesium, a metal, gives up two electrons to. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. The burning of magnesium metal reacts with oxygen found in the.
Using Lewis Symbols Diagram the Reaction Between Magnesium and Oxygen
The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The burning of magnesium metal reacts with oxygen found in the. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged.
Formation of magnesium oxide from magnesium and oxygen Brainly.in
The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). The burning of magnesium metal reacts with oxygen found in the. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. This is because magnesium (mg) loses 2. When the reaction with oxygen occurs, these two electrons are donated by.
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen
Yes, oxygen and magnesium form an ionic compound called magnesium oxide. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. This occurs when magnesium loses. This is because magnesium (mg) loses 2. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo.
Bonding Basics
Magnesium, a metal, gives up two electrons to. This is because magnesium (mg) loses 2. The burning of magnesium metal reacts with oxygen found in the. This occurs when magnesium loses. Yes, oxygen and magnesium form an ionic compound called magnesium oxide.
Making Molecules First Look at the Periodic Table
Magnesium, a metal, gives up two electrons to. This occurs when magnesium loses. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Yes, oxygen and magnesium form an ionic compound called magnesium oxide.
When magnesium makes an ionic bond with oxygen it loses two electrons
The burning of magnesium metal reacts with oxygen found in the. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. When the reaction with oxygen occurs, these two electrons are donated by the.
Reaction Magnesium and Oxygen ThinkTac Science Experiment YouTube
This is because magnesium (mg) loses 2. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. The burning of magnesium metal reacts with oxygen found in the. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium, a metal, gives up two electrons to.
Which diagram shows the correct way to represent an ionic compound of
This is because magnesium (mg) loses 2. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The burning of magnesium metal reacts with oxygen found in the. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. Magnesium, a metal, gives up two electrons to.
magnesium oxygen reaction
Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. This is because magnesium (mg) loses 2. Magnesium, a metal, gives up two electrons to. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −.
Magnesium Has A Charge Of 2 +, While Oxygen Has A Charge Of 2 −.
This occurs when magnesium loses. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). Magnesium, a metal, gives up two electrons to.
The Ionic Compound That Forms From Magnesium And Oxygen Is Magnesium Oxide, Which Has The Formula Mgo.
This is because magnesium (mg) loses 2. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. The burning of magnesium metal reacts with oxygen found in the. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo.