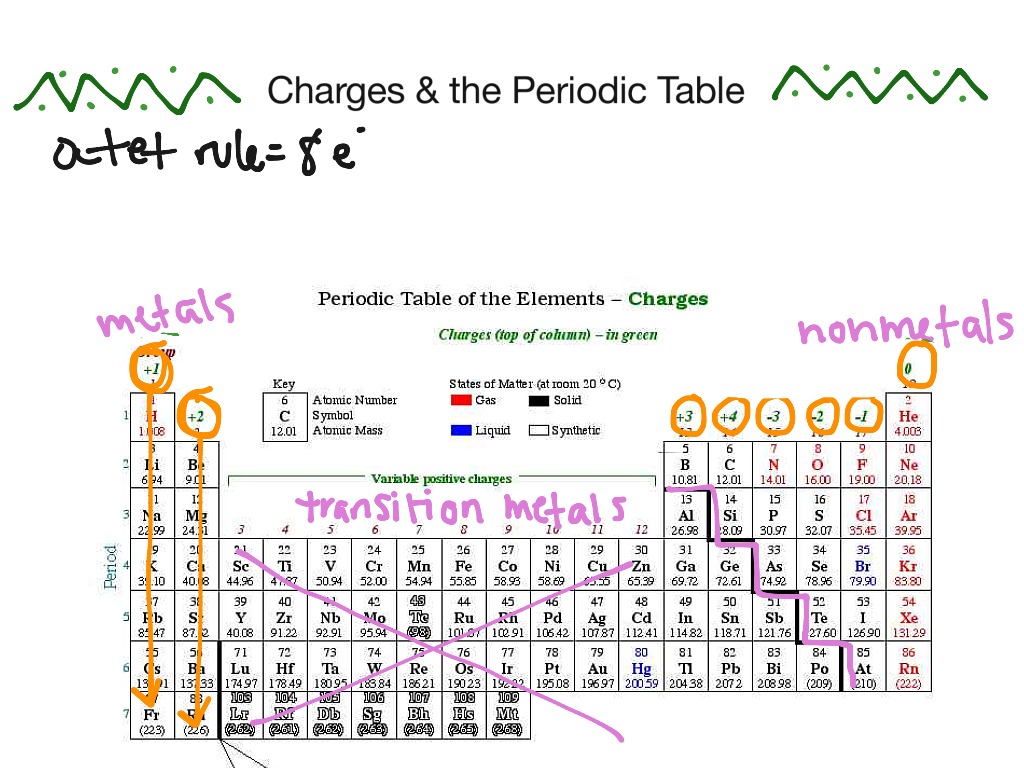
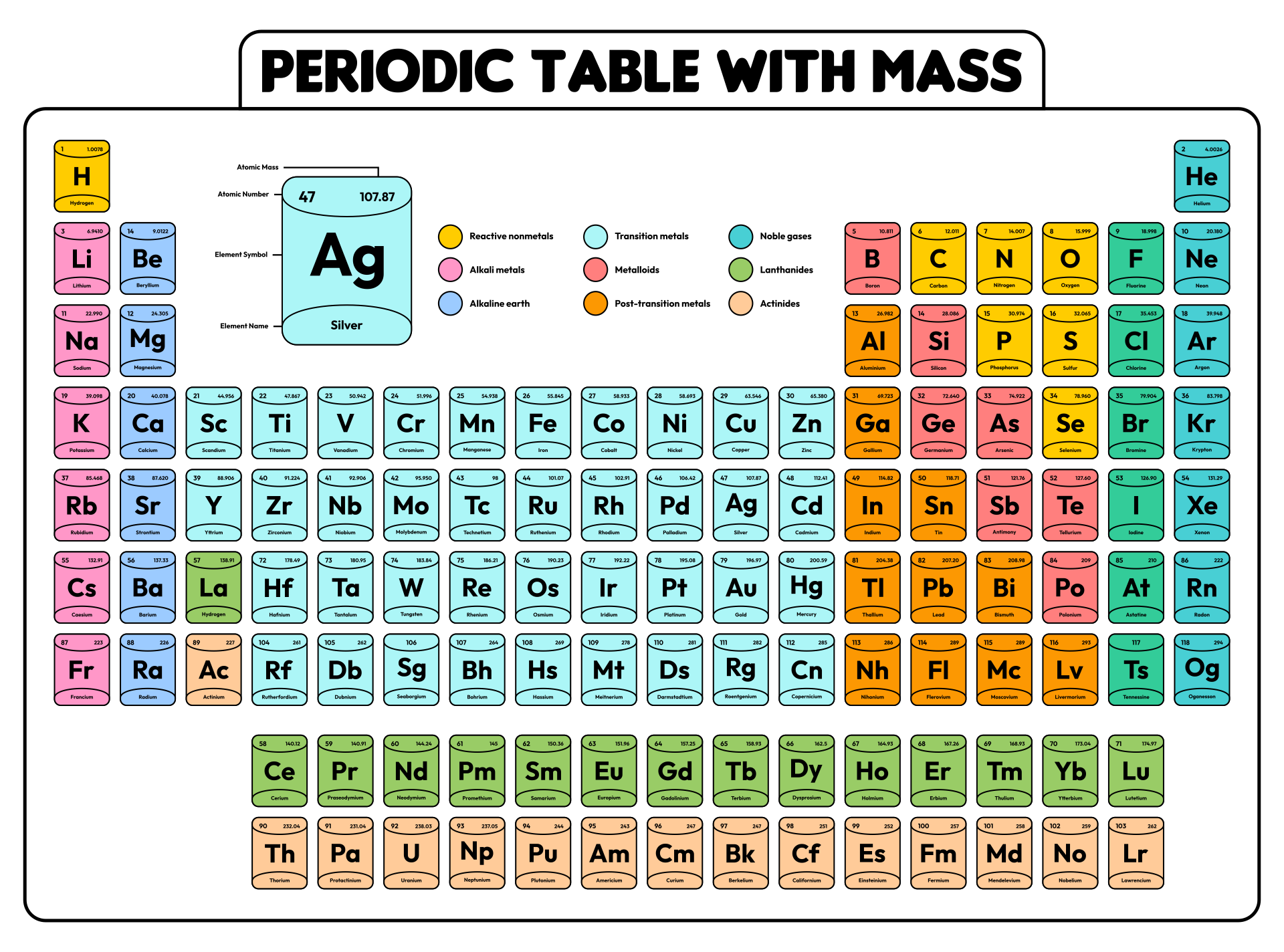
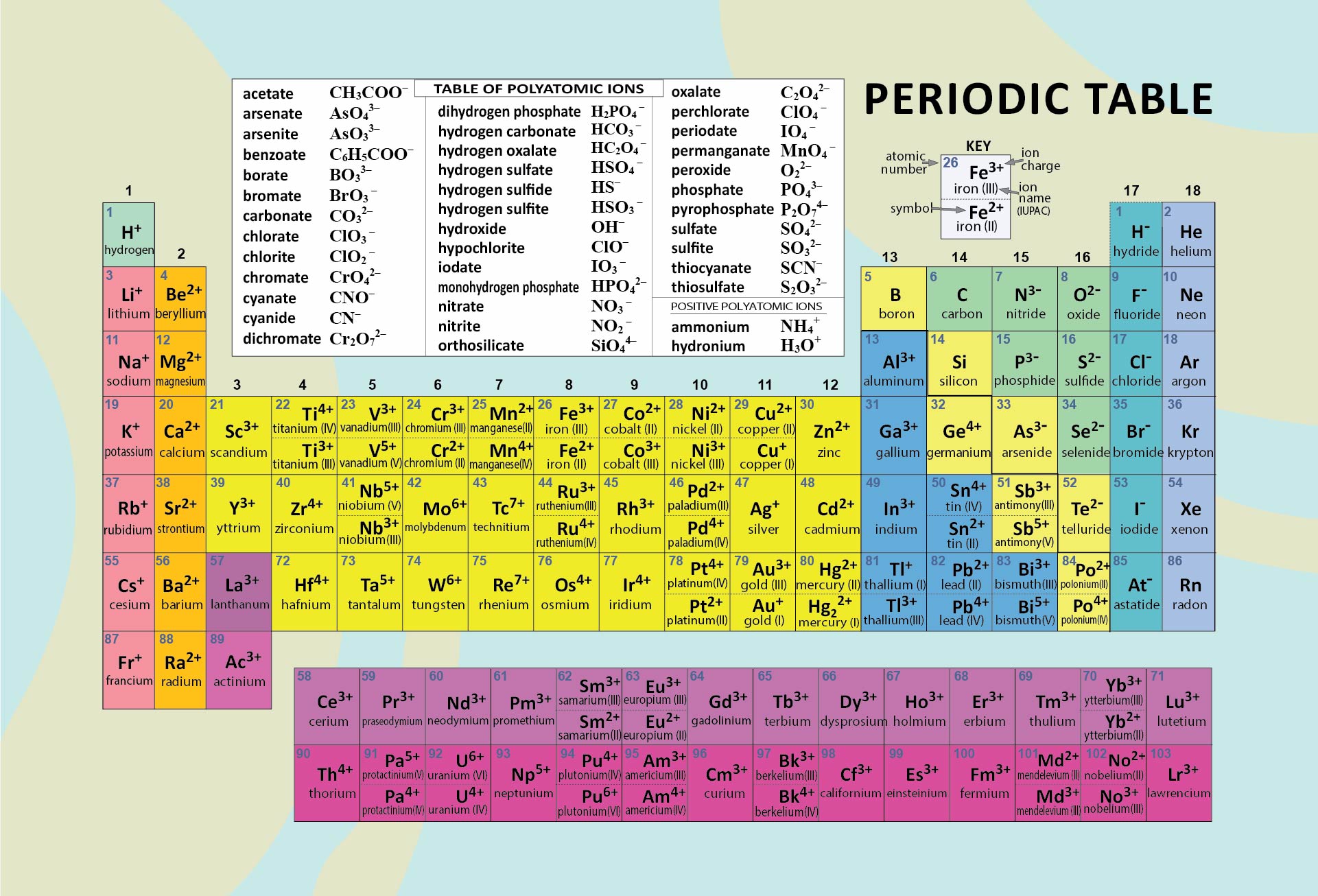
Periodic Table With Charges
Periodic Table With Charges - Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms gain or lose valence electrons to become more stable. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. These elements form cations with varying extents of charge. A period is the collection of elements that are arranged in a.
A period is the collection of elements that are arranged in a. Atoms gain or lose valence electrons to become more stable. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. These elements form cations with varying extents of charge. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions.
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. These elements form cations with varying extents of charge. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms gain or lose valence electrons to become more stable. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. A period is the collection of elements that are arranged in a.
Periodic Table with Charges 118 Elements
These elements form cations with varying extents of charge. Atoms gain or lose valence electrons to become more stable. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. A period is the collection of elements that are arranged in a. Charges of +2 or +3 are common, but charges ranging from.
Printable Periodic Table with Charges 2015
These elements form cations with varying extents of charge. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms gain or lose valence electrons to become more stable. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. A period is the collection of.
Printable Periodic Table Element Charges
Atoms gain or lose valence electrons to become more stable. These elements form cations with varying extents of charge. A period is the collection of elements that are arranged in a. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Charges of +2 or +3 are common, but charges ranging from.
Free Printable Periodic Table with Charges of Elements [PDF]
Atoms gain or lose valence electrons to become more stable. A period is the collection of elements that are arranged in a. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms are neutral, so i'll.
Downloadable Periodic Table Element Charges
The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Atoms gain or lose valence electrons to.
Periodic Table Labeled With Charges Periodic Table Timeline
These elements form cations with varying extents of charge. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms gain or lose valence electrons to become more stable. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. The periodic table is a tabular.
Downloadable Periodic Table Element Charges
A period is the collection of elements that are arranged in a. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms gain or lose valence electrons to become more stable. The periodic table.
Periodic Table With Names Of Elements And Charges
Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms gain or lose valence electrons to become more stable. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons.
Periodic Table Of Elements 10 Free PDF Printables Printablee
These elements form cations with varying extents of charge. Atoms gain or lose valence electrons to become more stable. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. A period is the collection of elements that.
Periodic Table with Charges PDF for Printing
These elements form cations with varying extents of charge. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number. Atoms gain or lose valence electrons to become more stable. Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. A period is.
A Period Is The Collection Of Elements That Are Arranged In A.
Atoms are neutral, so i'll assume you mean the charges formed when atoms lose or gain electrons to form ions. Charges of +2 or +3 are common, but charges ranging from +1 to +6 are. Atoms gain or lose valence electrons to become more stable. The periodic table is a tabular arrangement of the chemical elements in the increasing order of atomic number.



![Free Printable Periodic Table with Charges of Elements [PDF]](https://iperiodictable.com/wp-content/uploads/2020/12/Periodic-Table-with-Charges-1536x864.png)