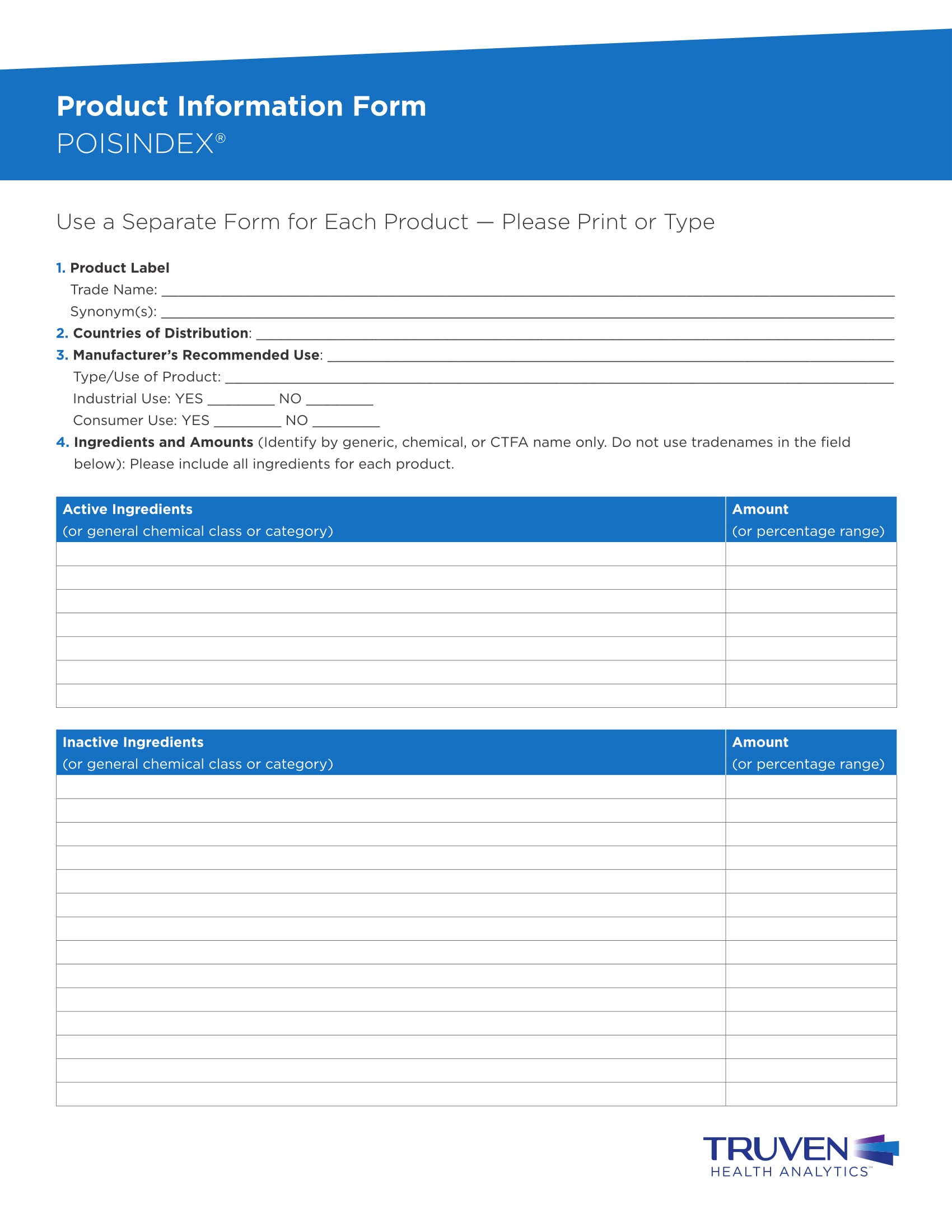
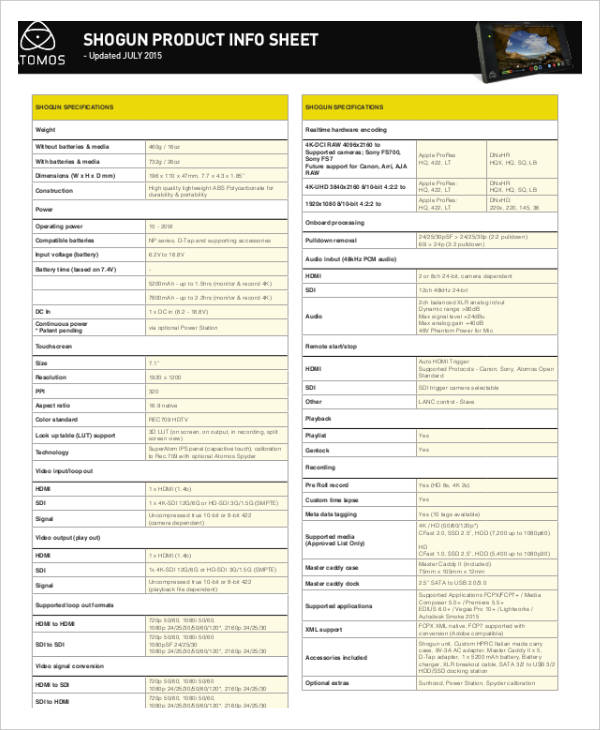
Templatesema Product Information Templates
Templatesema Product Information Templates - The european medicines agency (ema) makes guidance and templates available to provide. On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) has introduced a number of changes to. The european medicines agency's (ema) working group on quality review of documents (qrd). Standard statements are given in the template, which must be used whenever they are. Ema’s template builder allows you to create custom product templates easily and.
The european medicines agency (ema) makes guidance and templates available to provide. Ema’s template builder allows you to create custom product templates easily and. On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency's (ema) working group on quality review of documents (qrd). The european medicines agency (ema) has introduced a number of changes to. Standard statements are given in the template, which must be used whenever they are.
Standard statements are given in the template, which must be used whenever they are. The european medicines agency's (ema) working group on quality review of documents (qrd). On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) makes guidance and templates available to provide. Ema’s template builder allows you to create custom product templates easily and. The european medicines agency (ema) has introduced a number of changes to.
Ema Product Information Templates
Ema’s template builder allows you to create custom product templates easily and. Standard statements are given in the template, which must be used whenever they are. The european medicines agency (ema) has introduced a number of changes to. The european medicines agency (ema) makes guidance and templates available to provide. The european medicines agency's (ema) working group on quality review.
Ema Product Information Templates
The european medicines agency (ema) makes guidance and templates available to provide. Standard statements are given in the template, which must be used whenever they are. The european medicines agency (ema) has introduced a number of changes to. On 10 june 2015, the european medicines agency published the revised human product. Ema’s template builder allows you to create custom product.
Ema Product Information Templates
The european medicines agency (ema) has introduced a number of changes to. On 10 june 2015, the european medicines agency published the revised human product. Ema’s template builder allows you to create custom product templates easily and. The european medicines agency (ema) makes guidance and templates available to provide. Standard statements are given in the template, which must be used.
Ema Product Information Templates
The european medicines agency (ema) has introduced a number of changes to. On 10 june 2015, the european medicines agency published the revised human product. Standard statements are given in the template, which must be used whenever they are. The european medicines agency (ema) makes guidance and templates available to provide. Ema’s template builder allows you to create custom product.
Ema Product Information Templates
The european medicines agency's (ema) working group on quality review of documents (qrd). Standard statements are given in the template, which must be used whenever they are. The european medicines agency (ema) makes guidance and templates available to provide. On 10 june 2015, the european medicines agency published the revised human product. Ema’s template builder allows you to create custom.
11+ Product Sheet Templates Free Sample, Example Format Download Free & Premium Templates
The european medicines agency (ema) makes guidance and templates available to provide. The european medicines agency (ema) has introduced a number of changes to. Ema’s template builder allows you to create custom product templates easily and. Standard statements are given in the template, which must be used whenever they are. On 10 june 2015, the european medicines agency published the.
Ema Product Information Templates Printable Word Searches
The european medicines agency (ema) makes guidance and templates available to provide. Ema’s template builder allows you to create custom product templates easily and. On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) has introduced a number of changes to. The european medicines agency's (ema) working group on quality review of.
Ema Product Information Templates
On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) makes guidance and templates available to provide. The european medicines agency's (ema) working group on quality review of documents (qrd). The european medicines agency (ema) has introduced a number of changes to. Ema’s template builder allows you to create custom product templates.
Ema Product Information Templates
The european medicines agency (ema) has introduced a number of changes to. Ema’s template builder allows you to create custom product templates easily and. Standard statements are given in the template, which must be used whenever they are. On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) makes guidance and templates.
Ema Product Information Templates
Ema’s template builder allows you to create custom product templates easily and. Standard statements are given in the template, which must be used whenever they are. The european medicines agency (ema) makes guidance and templates available to provide. On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) has introduced a number.
The European Medicines Agency's (Ema) Working Group On Quality Review Of Documents (Qrd).
On 10 june 2015, the european medicines agency published the revised human product. The european medicines agency (ema) has introduced a number of changes to. Ema’s template builder allows you to create custom product templates easily and. Standard statements are given in the template, which must be used whenever they are.