The Formation Of A Positive Ion
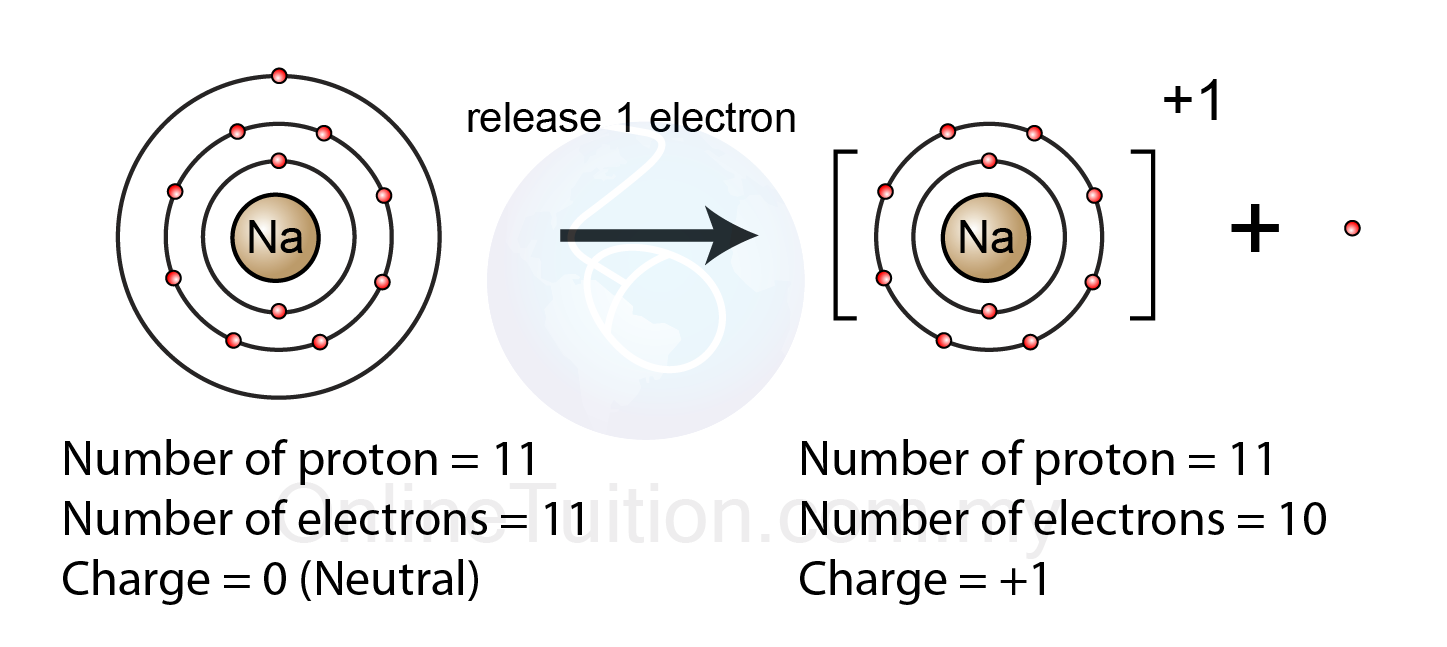
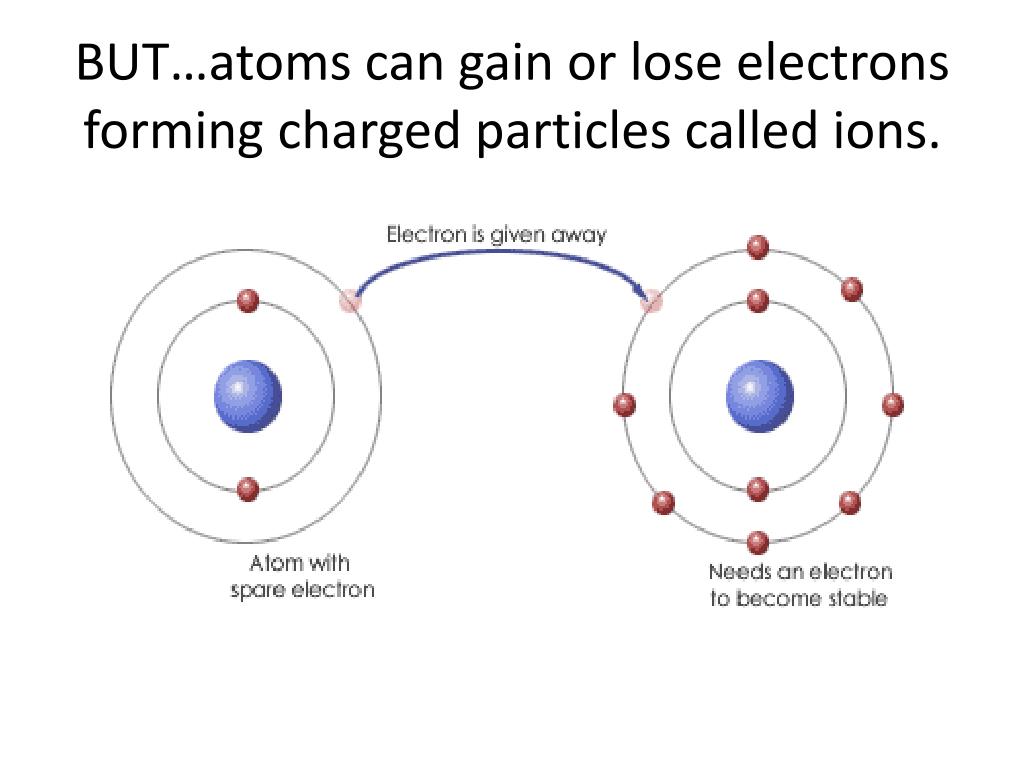
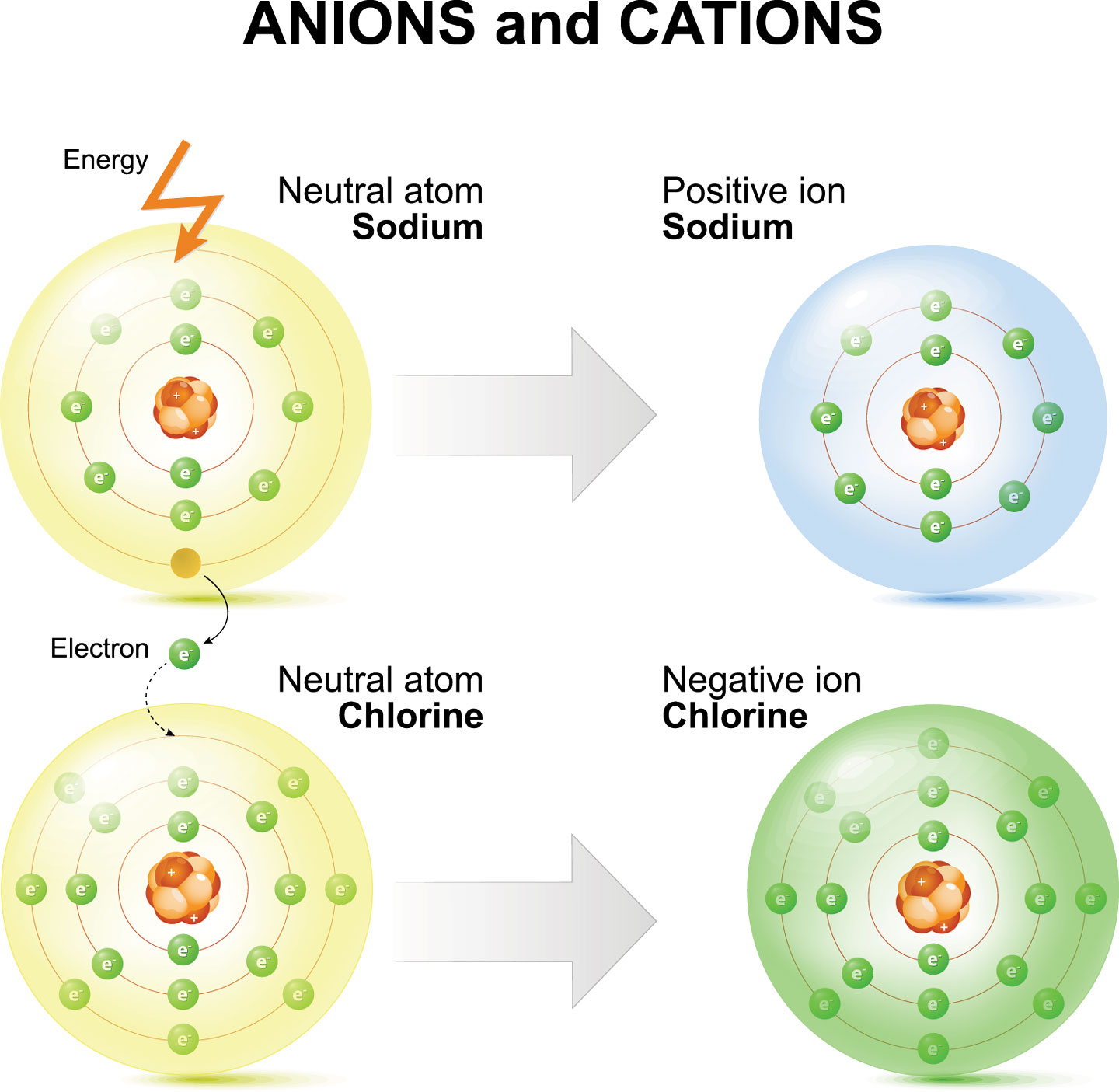
The Formation Of A Positive Ion - Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons. Positive and negative ions of certain elements can be created depending on the number of electrons in their structure.
Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons. Positive and negative ions of certain elements can be created depending on the number of electrons in their structure.
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
Forming Ions GCSE Chemistry Revision
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
NEW MAGNESIUM PERIODIC TABLE PROTONS Periodic
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
Air ions formation. diagram. Oxygen atoms Stock Vector by ©edesignua
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
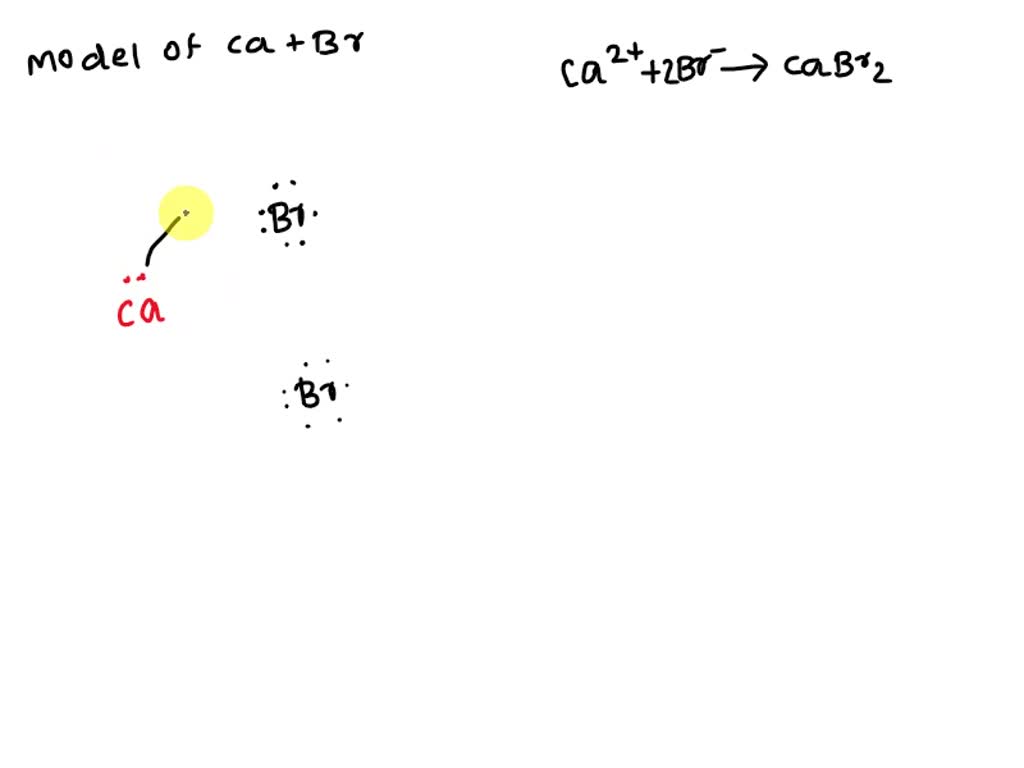
draw models to represent the formation of the positive calcium ion and
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
Anion_Formation
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
draw models to represent the formation of the positive calcium ion and
Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons. Positive and negative ions of certain elements can be created depending on the number of electrons in their structure.
Formation of ions IGCSE Chemistry Revision Notes
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
Formation of positive ion YouTube
Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons. Positive and negative ions of certain elements can be created depending on the number of electrons in their structure.
PPT Ion Formation PowerPoint Presentation, free download ID2508414
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
Explainer Ions and radicals in our world Science News for Students
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure. Positive ions are called cations and form when atoms lose electrons, meaning they have more protons than electrons.
Positive Ions Are Called Cations And Form When Atoms Lose Electrons, Meaning They Have More Protons Than Electrons.
Positive and negative ions of certain elements can be created depending on the number of electrons in their structure.