What Does The Electron Sea Model For Metals Suggest
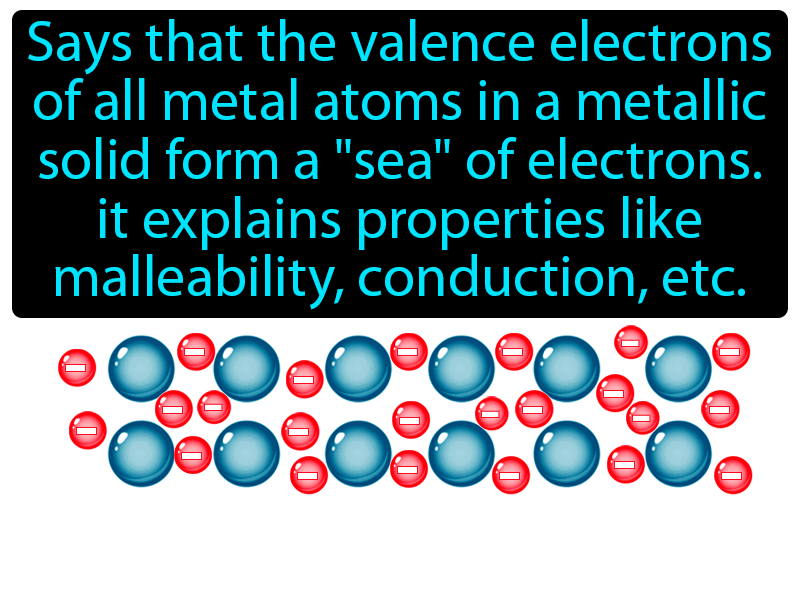
What Does The Electron Sea Model For Metals Suggest - Why don't metals break when. The electron sea model for metals suggests that valence electrons are delocalized. The electron sea model for metals suggests that valence electrons are delocalized and drift. What does the electron sea model for metals suggest? The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. In electron sea model, the valence electrons in metals are delocalized instead of.
The electron sea model for metals suggests that valence electrons are delocalized and drift. Why don't metals break when. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. In electron sea model, the valence electrons in metals are delocalized instead of. What does the electron sea model for metals suggest? The electron sea model for metals suggests that valence electrons are delocalized.
The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. Why don't metals break when. The electron sea model for metals suggests that valence electrons are delocalized. The electron sea model for metals suggests that valence electrons are delocalized and drift. In electron sea model, the valence electrons in metals are delocalized instead of. What does the electron sea model for metals suggest?
SOLUTION Free electron theory electron sea model Studypool
The electron sea model for metals suggests that valence electrons are delocalized. What does the electron sea model for metals suggest? The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. Why don't metals break when. In electron sea model, the valence electrons in metals are delocalized instead of.
Definition Of Electron Sea Model DEFINITIONVA
Why don't metals break when. What does the electron sea model for metals suggest? The electron sea model for metals suggests that valence electrons are delocalized and drift. The electron sea model for metals suggests that valence electrons are delocalized. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded.
Metallic Bonding Definition, Models & Properties Lesson
The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. In electron sea model, the valence electrons in metals are delocalized instead of. Why don't metals break when. The electron sea model for metals suggests that valence electrons are delocalized. What does the electron sea model for metals suggest?
Structure Metallic Bond Electron Sea Model Stock Vector (Royalty Free
What does the electron sea model for metals suggest? The electron sea model for metals suggests that valence electrons are delocalized and drift. The electron sea model for metals suggests that valence electrons are delocalized. Why don't metals break when. In electron sea model, the valence electrons in metals are delocalized instead of.
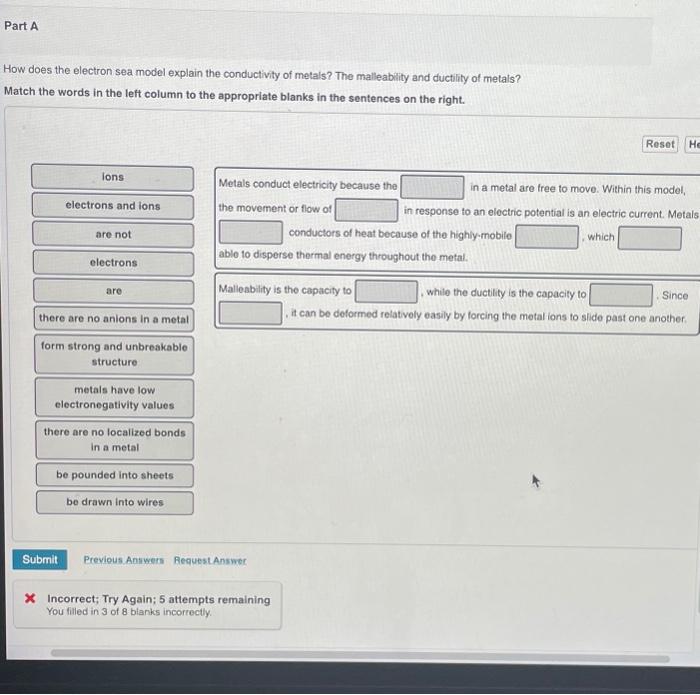
Solved Part A How does the electron sea model explain the
What does the electron sea model for metals suggest? In electron sea model, the valence electrons in metals are delocalized instead of. The electron sea model for metals suggests that valence electrons are delocalized and drift. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. The electron sea model for metals suggests that valence.
Electron Sea Model To account for bounding in metals Lorentz propose o wo..
What does the electron sea model for metals suggest? The electron sea model for metals suggests that valence electrons are delocalized and drift. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. In electron sea model, the valence electrons in metals are delocalized instead of. The electron sea model for metals suggests that valence.
Electron Sea Model Definition & Image GameSmartz
The electron sea model for metals suggests that valence electrons are delocalized. What does the electron sea model for metals suggest? Why don't metals break when. In electron sea model, the valence electrons in metals are delocalized instead of. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded.
SOLVEDHow does the electron sea model explain the conductivity of
Why don't metals break when. In electron sea model, the valence electrons in metals are delocalized instead of. The electron sea model for metals suggests that valence electrons are delocalized and drift. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. The electron sea model for metals suggests that valence electrons are delocalized.
SOLVEDHow does the electronsea model account for the electrical and
Why don't metals break when. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. The electron sea model for metals suggests that valence electrons are delocalized. What does the electron sea model for metals suggest? In electron sea model, the valence electrons in metals are delocalized instead of.
What Is the ElectronSea Model?
The electron sea model for metals suggests that valence electrons are delocalized and drift. In electron sea model, the valence electrons in metals are delocalized instead of. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. The electron sea model for metals suggests that valence electrons are delocalized. Why don't metals break when.
Why Don't Metals Break When.
The electron sea model for metals suggests that valence electrons are delocalized and drift. The 'sea of electrons' model explains metallic bonding as a lattice of positive ions surrounded. What does the electron sea model for metals suggest? In electron sea model, the valence electrons in metals are delocalized instead of.







:max_bytes(150000):strip_icc()/abstract-shape-made-of-metallic-spheres-rising-up-from-a-silver-tray-859619196-5b82ab8cc9e77c0057c91de3.jpg)