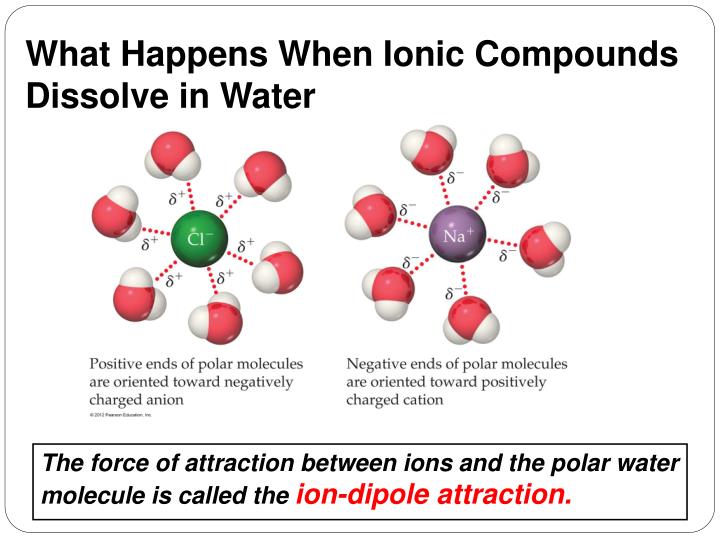
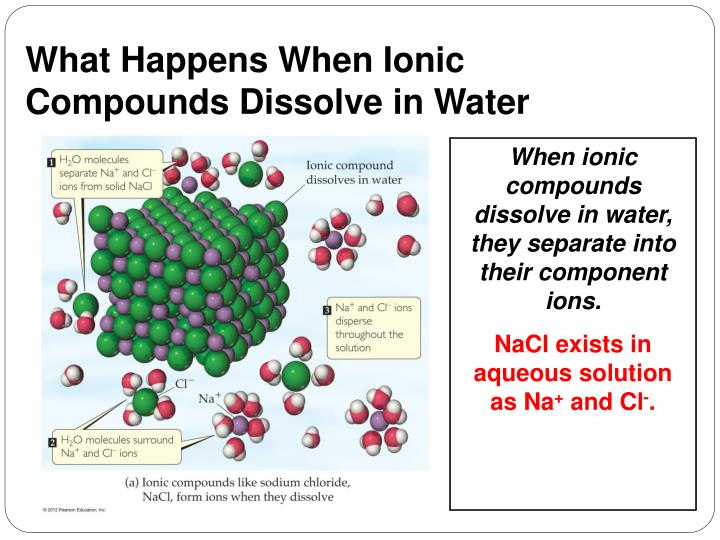
What Happens When Ionic Compounds Dissolve In Water
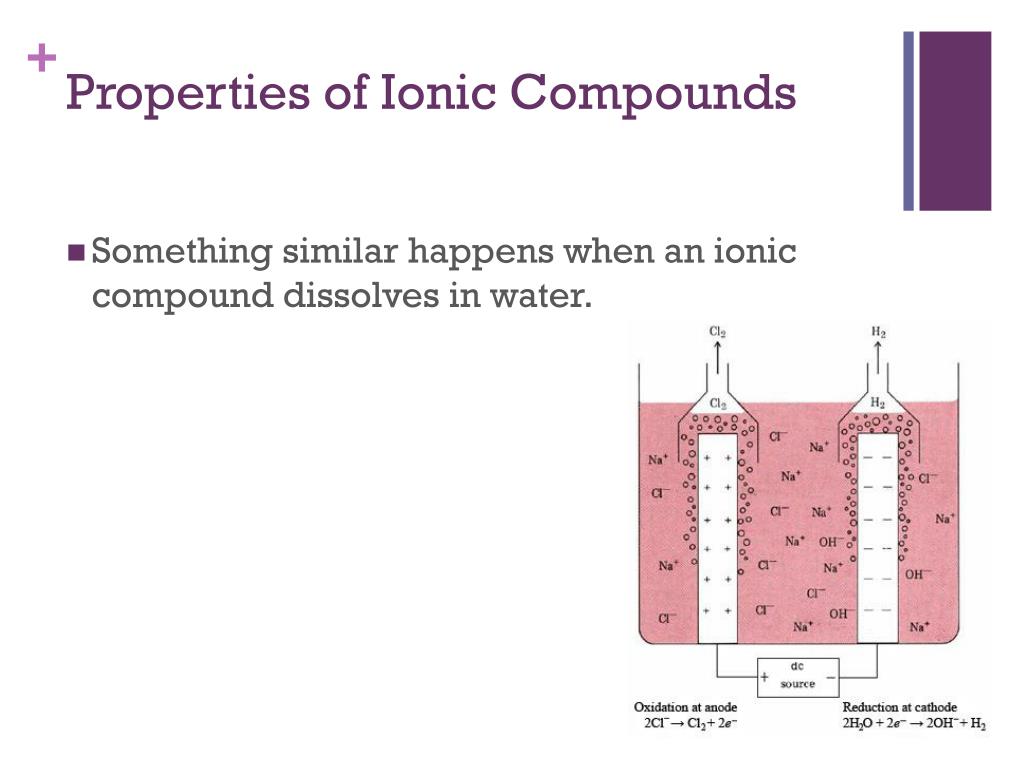
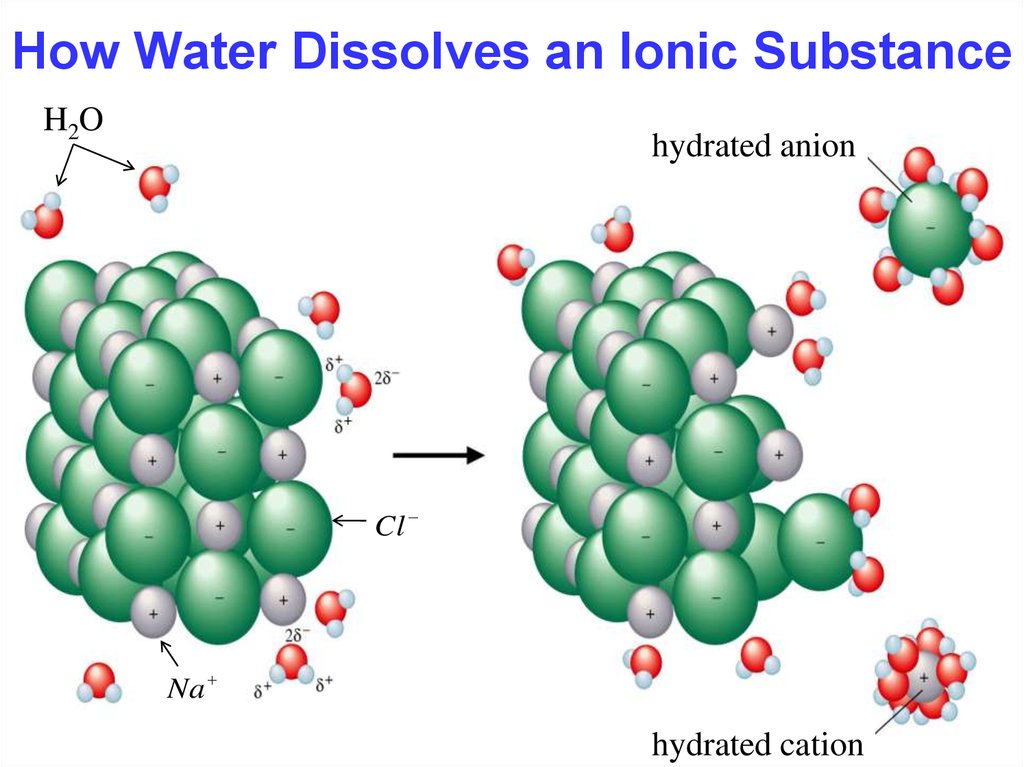
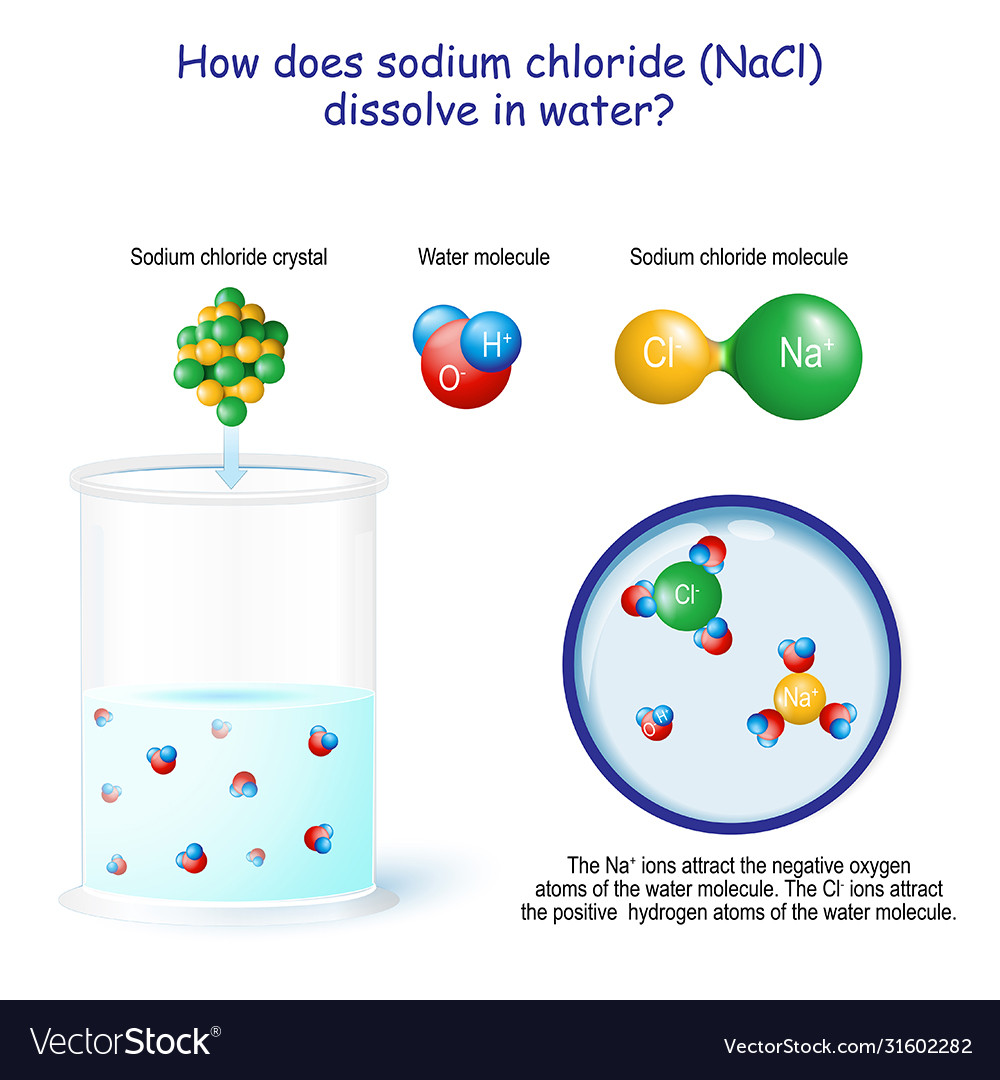
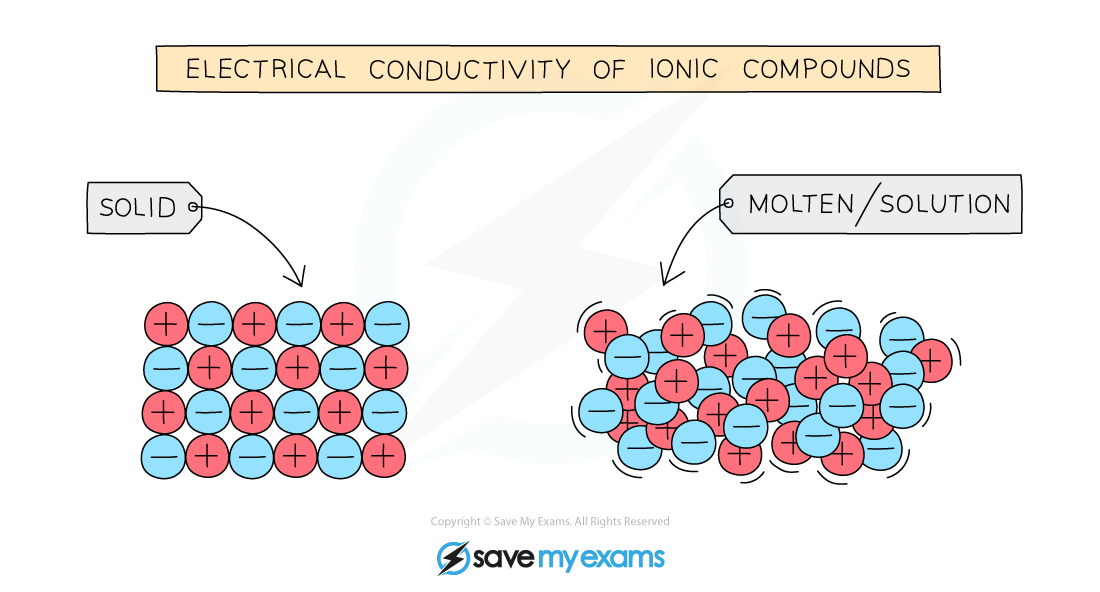
What Happens When Ionic Compounds Dissolve In Water - When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but. When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. Water is a polar compound that.
When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. Water is a polar compound that. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because.
Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. Water is a polar compound that. When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but.
What happens when an ionic compound dissolves in water How does
Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Water is a polar compound that. When ionic compounds dissolve in water, the ions in the solid.
Aqueous Solutions of Electrolytes online presentation
Water is a polar compound that. When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the.
SOLVED What happens when ionic compounds dissolve in water? a) Cations
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but. When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. Water is a polar.
What Happens When an Ionic Compound Dissolves in Water
When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Ionic compounds.
Diagram Of Salt Dissolved In Water
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but..
What Happens to Ionic & Covalent Compounds When They Dissolve in Water
Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Water is a polar compound that. When an ionic compound dissolves in water or an aqueous solution,.
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure
Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Water is a polar compound that. When an ionic compound dissolves in water or an aqueous solution,.
CK12Foundation
When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy.
PPT UNIT 5 PowerPoint Presentation ID2276550
When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Ionic compounds.
PPT UNIT 5 PowerPoint Presentation ID2276550
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but. Water is a polar compound that. Ionic compounds dissolve in water if the energy given off when the ions interact with water.
Ionic Compounds Dissolve In Water If The Energy Given Off When The Ions Interact With Water Molecules Compensates For The Energy Needed.
When an ionic compound dissolves in water or an aqueous solution, it dissociates into positive and negative ions. When ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. When an ionic compound dissolves in water, the individual cations and anions are completely surrounded by water molecules, but. Water is a polar compound that.