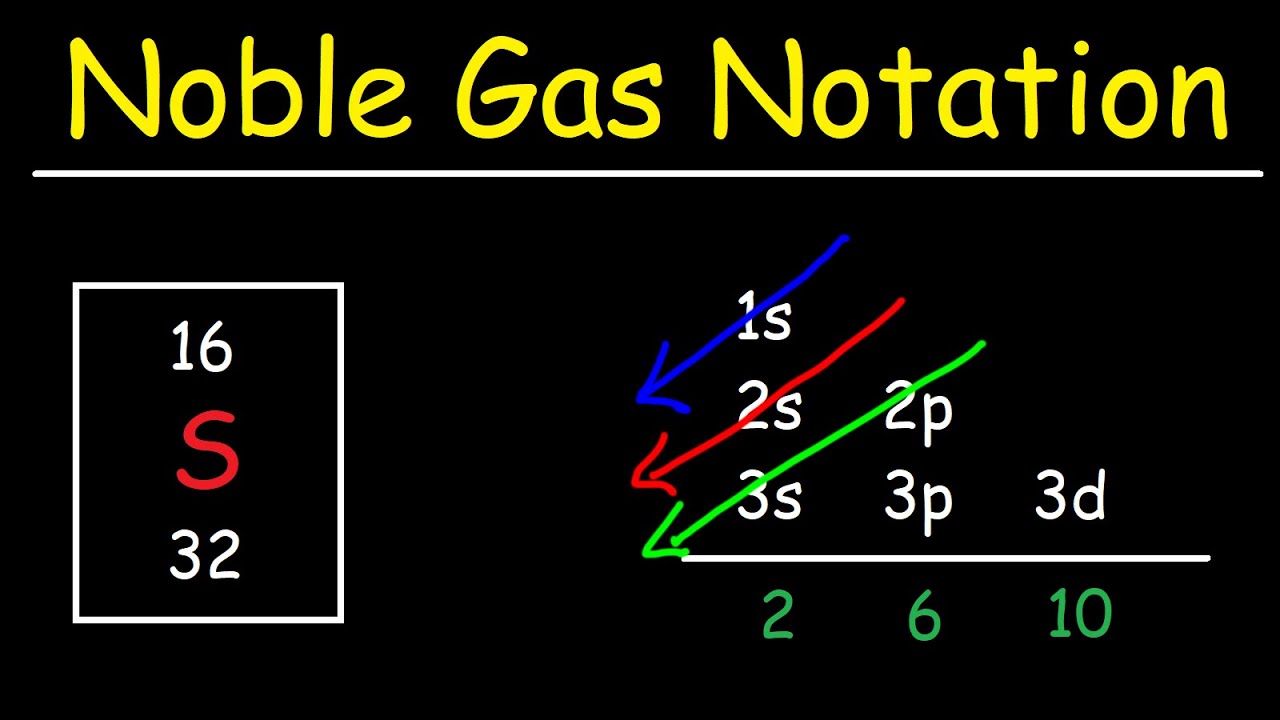
What Is Pseudo Noble Gas Configuration
What Is Pseudo Noble Gas Configuration - It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It is achieved when an atom. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a.
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. It is achieved when an atom.
Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration.
SOLVEDWhat is a pseudonoble gas configuration? Give an example of one
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or.
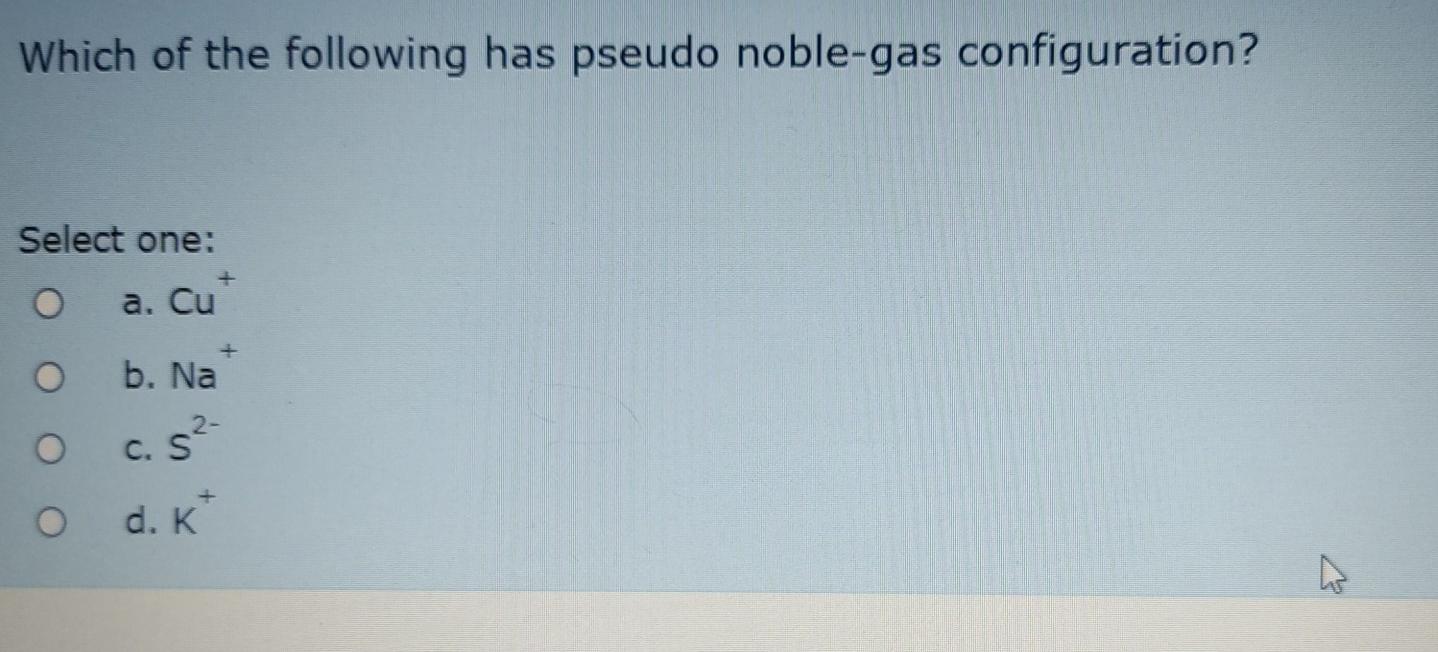
Solved Which of the following has pseudo noblegas
A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It is achieved when an atom. It refers to elements having 18 electrons instead.
SECTIONB 86. Pseudo inert gas configuration is Filo
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It is achieved when an atom. Yes, the electron structure of a zinc ion.
Antimony noble gas configuration virtmilitary
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p.
Noble Gas Configuration For Cobalt Ash in The Wild
It is achieved when an atom. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. A pseudo noble gas electron configuration refers to the electron.
Noble Gas Configuration Practice Questions
A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they.
44+ noble gas electron configuration calculator EileenSuzie
It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. Yes, the electron structure of a zinc ion.
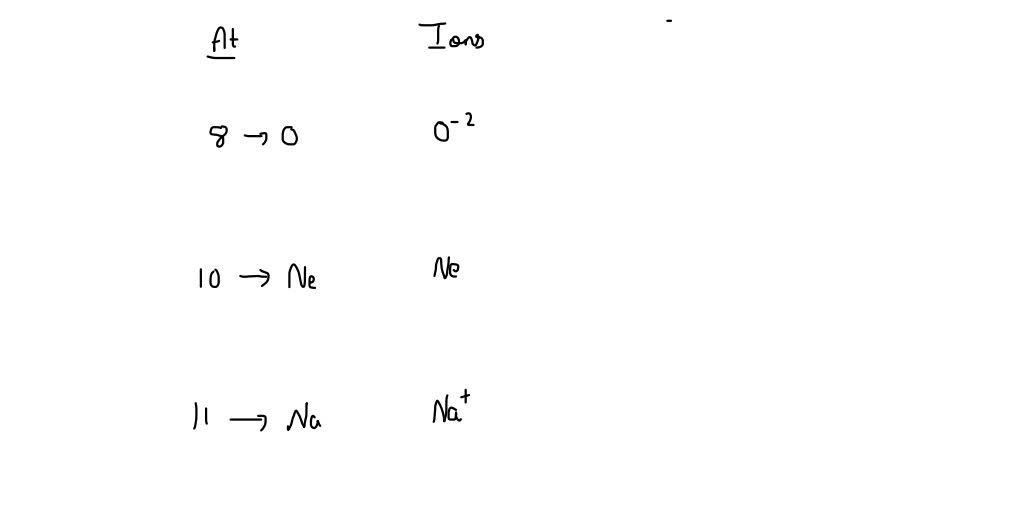
SOLVED Give the Pseudo Noble gas configuration if 8,10,11 ions
Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. It is achieved when an atom. A pseudo noble gas electron configuration refers to the electron.
Noble Gas Configuration Of Vanadium
Yes, the electron structure of a zinc ion (zn2+) achieves a pseudo noble gas configuration by losing two electrons to have a. It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble.
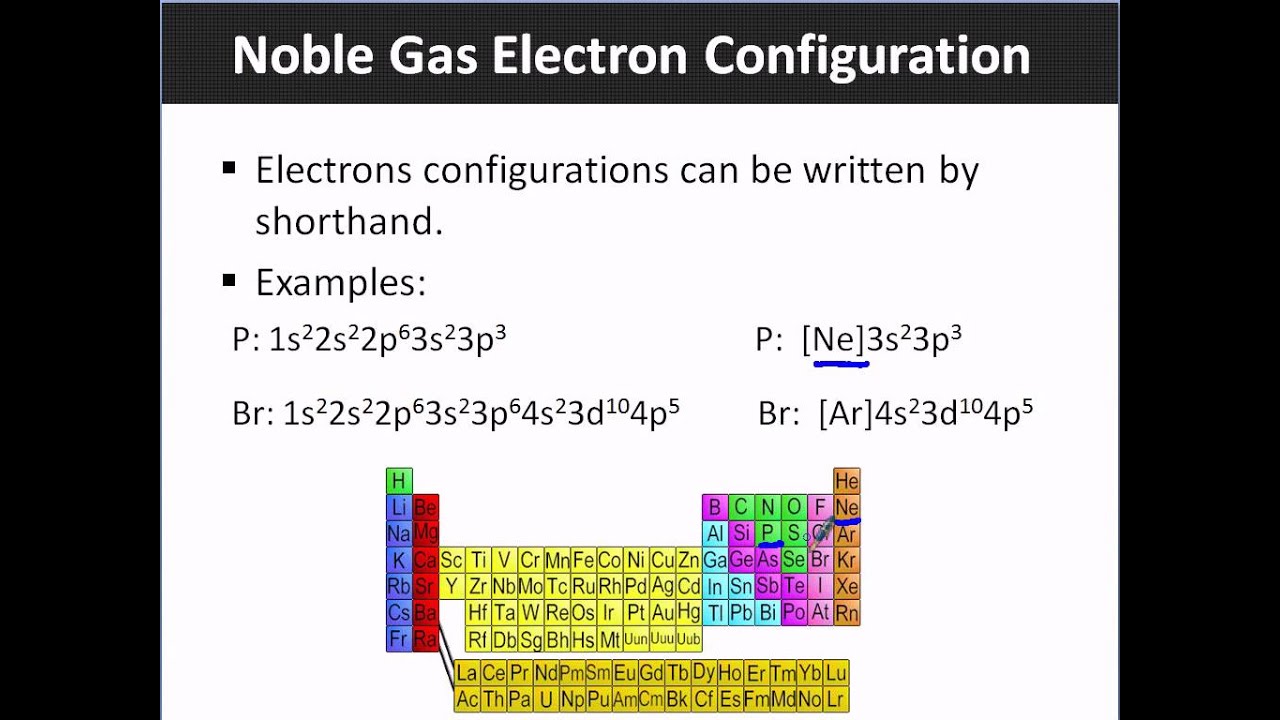
How to Write a Noble Gas Configuration for Atoms of an Element
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to.
Yes, The Electron Structure Of A Zinc Ion (Zn2+) Achieves A Pseudo Noble Gas Configuration By Losing Two Electrons To Have A.
It refers to elements having 18 electrons instead of just 8 in their outermost electron configuration when they lose or gain electrons. A pseudo noble gas electron configuration refers to the electron configuration of an atom where the outermost s and p orbitals are completely filled,. A pseudo noble gas configuration refers to an electron configuration that is similar to a noble gas configuration. It is achieved when an atom.