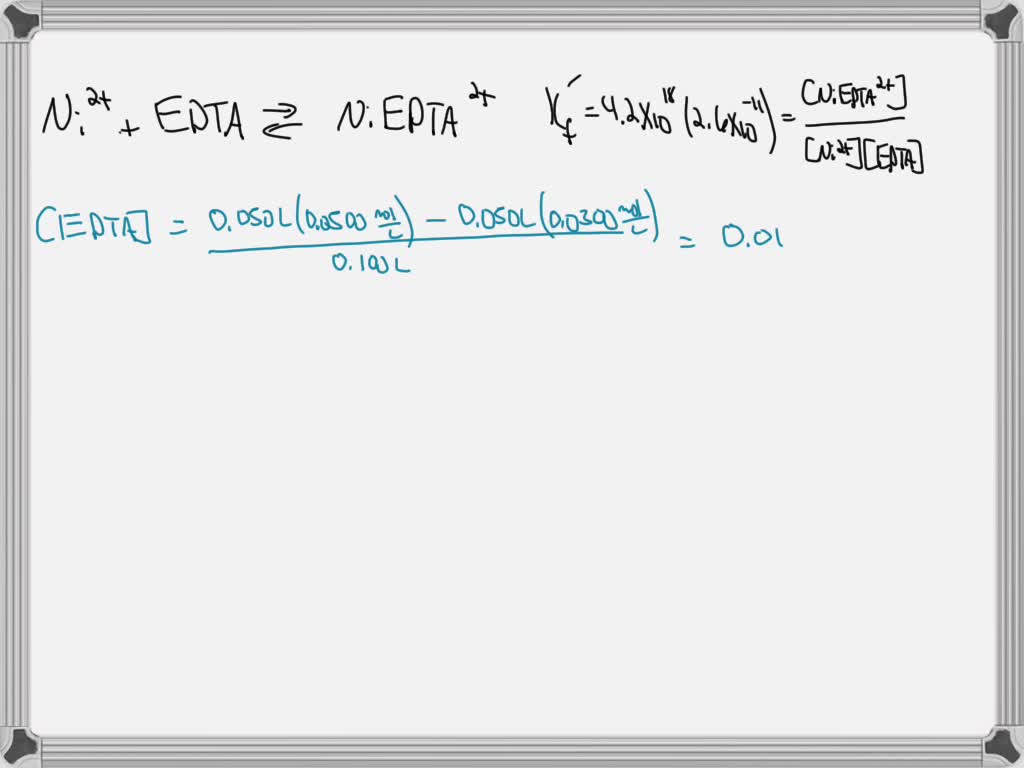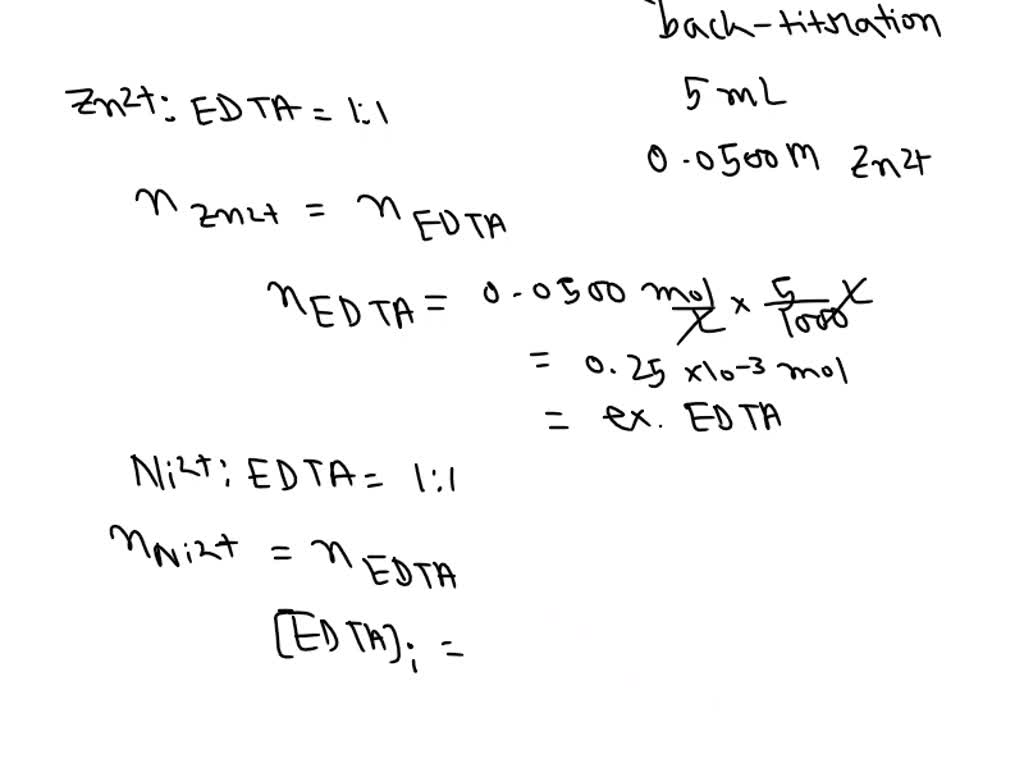What Is The Concentration Of Ni2 That Remains In Solution
What Is The Concentration Of Ni2 That Remains In Solution - What concentration of ni2+ remains in solution after electrolysis of 100 ml of 0.25 m niso4 solution when using a current of 2.40 amperes. What is the concentration of ni2+ that remains in solution? Express your answer in moles per liter to three significant figures. The value of kf for ni(nh3)62+ is. The concentration of ni2+ ions that remains after the reaction is 0.0117 m. After the solution reaches equilibrium, what concentration of ni2+(aq) remains? The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. To find the concentration of ni2+.
The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. What concentration of ni2+ remains in solution after electrolysis of 100 ml of 0.25 m niso4 solution when using a current of 2.40 amperes. The concentration of ni2+ ions that remains after the reaction is 0.0117 m. The value of kf for ni(nh3)62+ is. What is the concentration of ni2+ that remains in solution? To find the concentration of ni2+. After the solution reaches equilibrium, what concentration of ni2+(aq) remains? Express your answer in moles per liter to three significant figures.
What concentration of ni2+ remains in solution after electrolysis of 100 ml of 0.25 m niso4 solution when using a current of 2.40 amperes. To find the concentration of ni2+. What is the concentration of ni2+ that remains in solution? The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The value of kf for ni(nh3)62+ is. Express your answer in moles per liter to three significant figures. The concentration of ni2+ ions that remains after the reaction is 0.0117 m. After the solution reaches equilibrium, what concentration of ni2+(aq) remains?
Solved calculate the Ni2+ concentration of a solution made
Express your answer in moles per liter to three significant figures. What concentration of ni2+ remains in solution after electrolysis of 100 ml of 0.25 m niso4 solution when using a current of 2.40 amperes. The value of kf for ni(nh3)62+ is. What is the concentration of ni2+ that remains in solution? To find the concentration of ni2+.
SOLVED At a certain temperature, the concentration of Ni2+ ions in a
What is the concentration of ni2+ that remains in solution? To find the concentration of ni2+. The value of kf for ni(nh3)62+ is. The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The concentration of ni2+ ions that remains after the reaction is 0.0117 m.
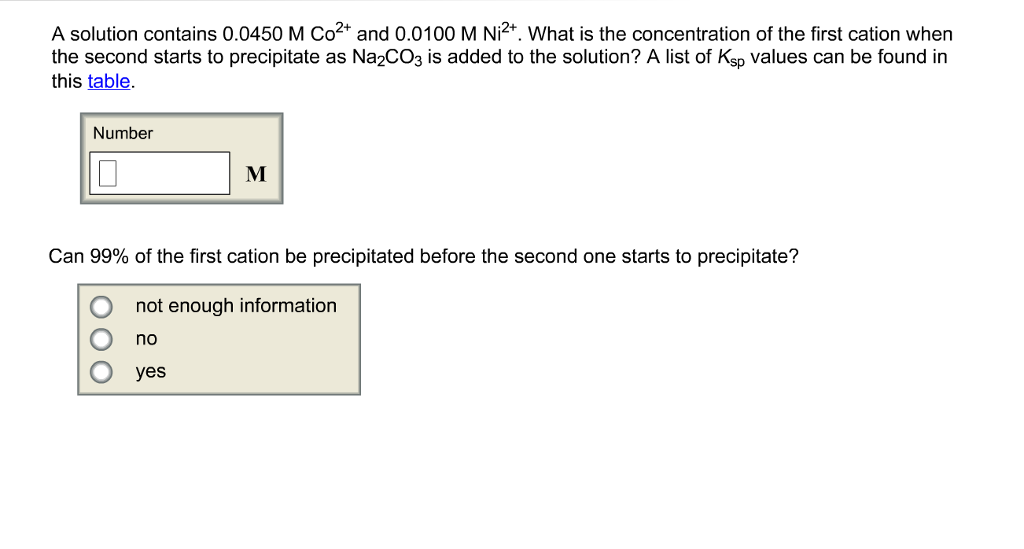
Solved A solution contains 0.0450 M Co2 and 0.0100 M Ni2+.
Express your answer in moles per liter to three significant figures. To find the concentration of ni2+. What is the concentration of ni2+ that remains in solution? The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. What concentration of ni2+ remains in solution after electrolysis of 100 ml of.
SOLVED Problem Calculate the concentration of Ni?+ in a solution that
What is the concentration of ni2+ that remains in solution? The value of kf for ni(nh3)62+ is. The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The concentration of ni2+ ions that remains after the reaction is 0.0117 m. What concentration of ni2+ remains in solution after electrolysis of.
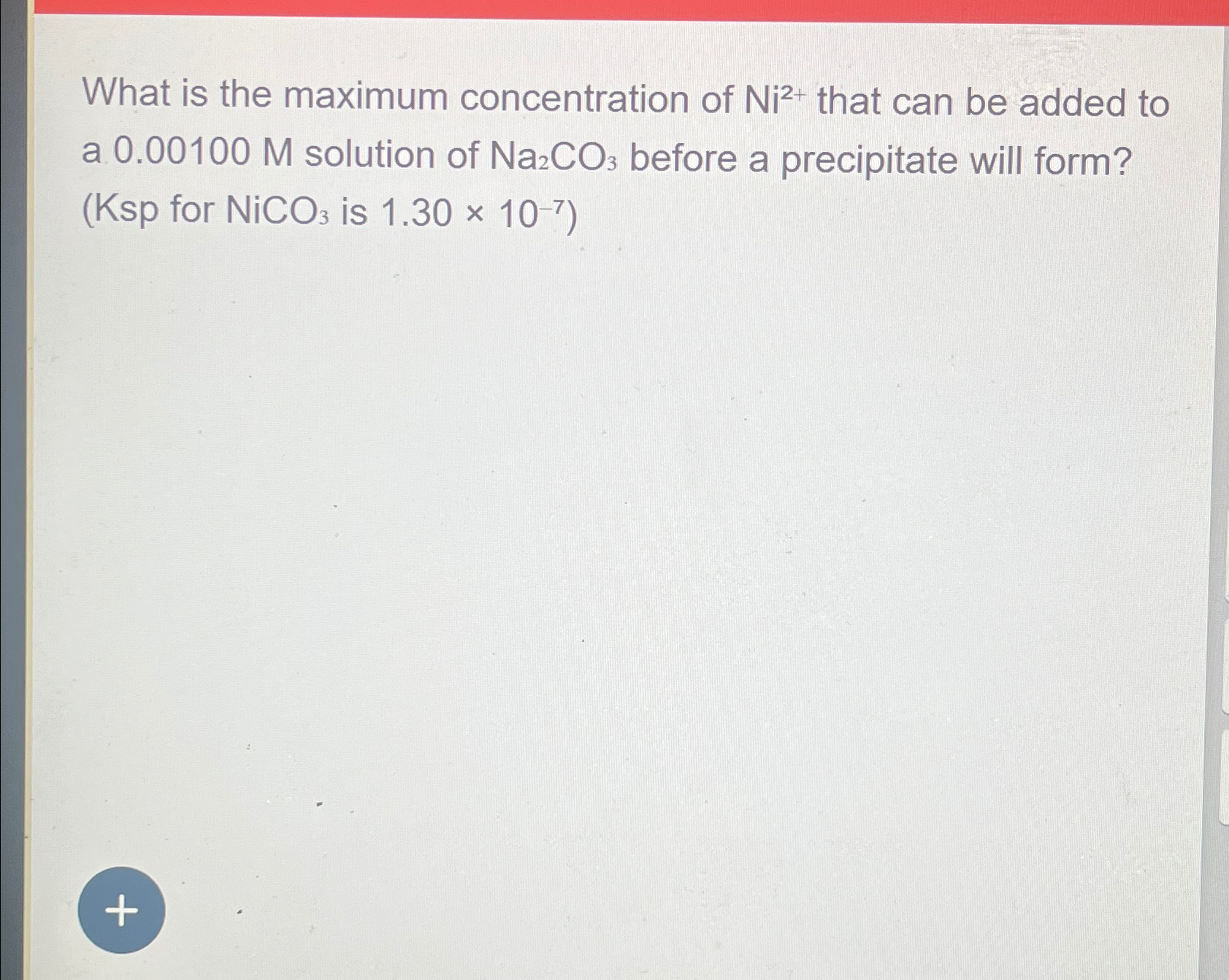
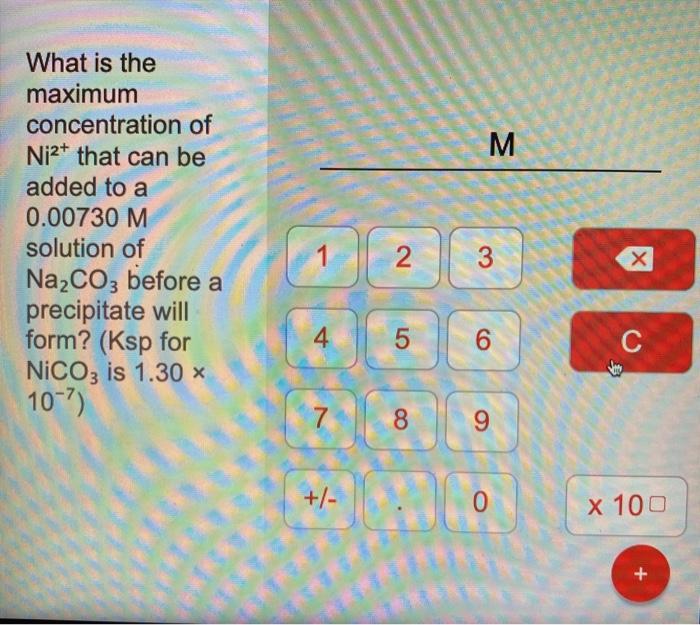
Solved What is the maximum concentration of Ni2+ that can
After the solution reaches equilibrium, what concentration of ni2+(aq) remains? The concentration of ni2+ ions that remains after the reaction is 0.0117 m. The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. What is the concentration of ni2+ that remains in solution? What concentration of ni2+ remains in solution.
4. Calculate the concentration of Ni²+ in... Physical Chemistry
The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The concentration of ni2+ ions that remains after the reaction is 0.0117 m. Express your answer in moles per liter to three significant figures. The value of kf for ni(nh3)62+ is. To find the concentration of ni2+.
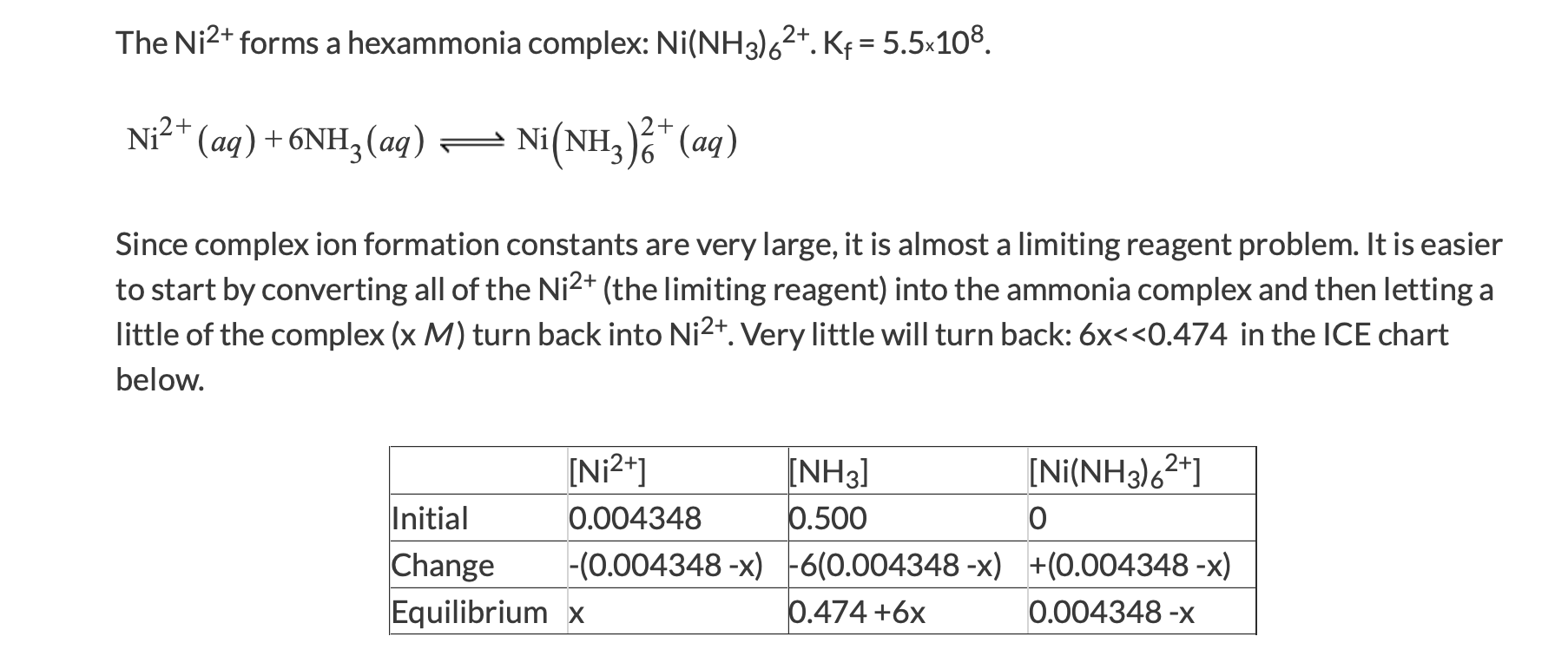
Solved Question What is the equilibrium concentration of
The concentration of ni2+ ions that remains after the reaction is 0.0117 m. To find the concentration of ni2+. Express your answer in moles per liter to three significant figures. The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The value of kf for ni(nh3)62+ is.
SOLVED Calculate the concentration of Ni2 in the solution prepared by
The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The value of kf for ni(nh3)62+ is. Express your answer in moles per liter to three significant figures. After the solution reaches equilibrium, what concentration of ni2+(aq) remains? To find the concentration of ni2+.
Solved M What is the maximum concentration of Ni2+ that can
The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. The concentration of ni2+ ions that remains after the reaction is 0.0117 m. After the solution reaches equilibrium, what concentration of ni2+(aq) remains? The value of kf for ni(nh3)62+ is. What concentration of ni2+ remains in solution after electrolysis of.
Solved Q was a solution of Ni2+ and a concentration cell was
Express your answer in moles per liter to three significant figures. The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. What is the concentration of ni2+ that remains in solution? After the solution reaches equilibrium, what concentration of ni2+(aq) remains? What concentration of ni2+ remains in solution after electrolysis.
What Is The Concentration Of Ni2+ That Remains In Solution?
The concentration of ni2+ ions that remains after the reaction is 0.0117 m. What concentration of ni2+ remains in solution after electrolysis of 100 ml of 0.25 m niso4 solution when using a current of 2.40 amperes. The concentration of ni2+ ions remaining in the solution can be found by applying faraday's first law of electrolysis and. After the solution reaches equilibrium, what concentration of ni2+(aq) remains?
Express Your Answer In Moles Per Liter To Three Significant Figures.
The value of kf for ni(nh3)62+ is. To find the concentration of ni2+.