What Is The Concentration Of X2 In A 0 150
What Is The Concentration Of X2 In A 0 150 - 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The concentration of x²⁻ in a 0.150. The option (a) is correct. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11.
For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The option (a) is correct. The concentration of x²⁻ in a 0.150. 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The concentration of x²⁻ is found to be 2.3×10⁻⁷ m.
The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The concentration of x²⁻ in a 0.150. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The option (a) is correct. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x?
Struggling to Stay Focused? Tips to Improve Concentration
1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The option (a) is correct. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ is found to be 2.3×10⁻⁷ m.
SOLVED What is the concentration in mEq/L of a Mg2+ solution that is
For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The option (a) is correct. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ in a 0.150.
How To Get Equilibrium Concentration
What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The option (a) is correct. 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11.
Concentration Images Of God
1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ in a 0.150. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The option (a) is correct.
Discriminating Concentration Bioassays Vector SVG Icon SVG Repo
The concentration of x²⁻ in a 0.150. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The option (a) is correct. 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The concentration of x²⁻ is found to be 2.3×10⁻⁷ m.
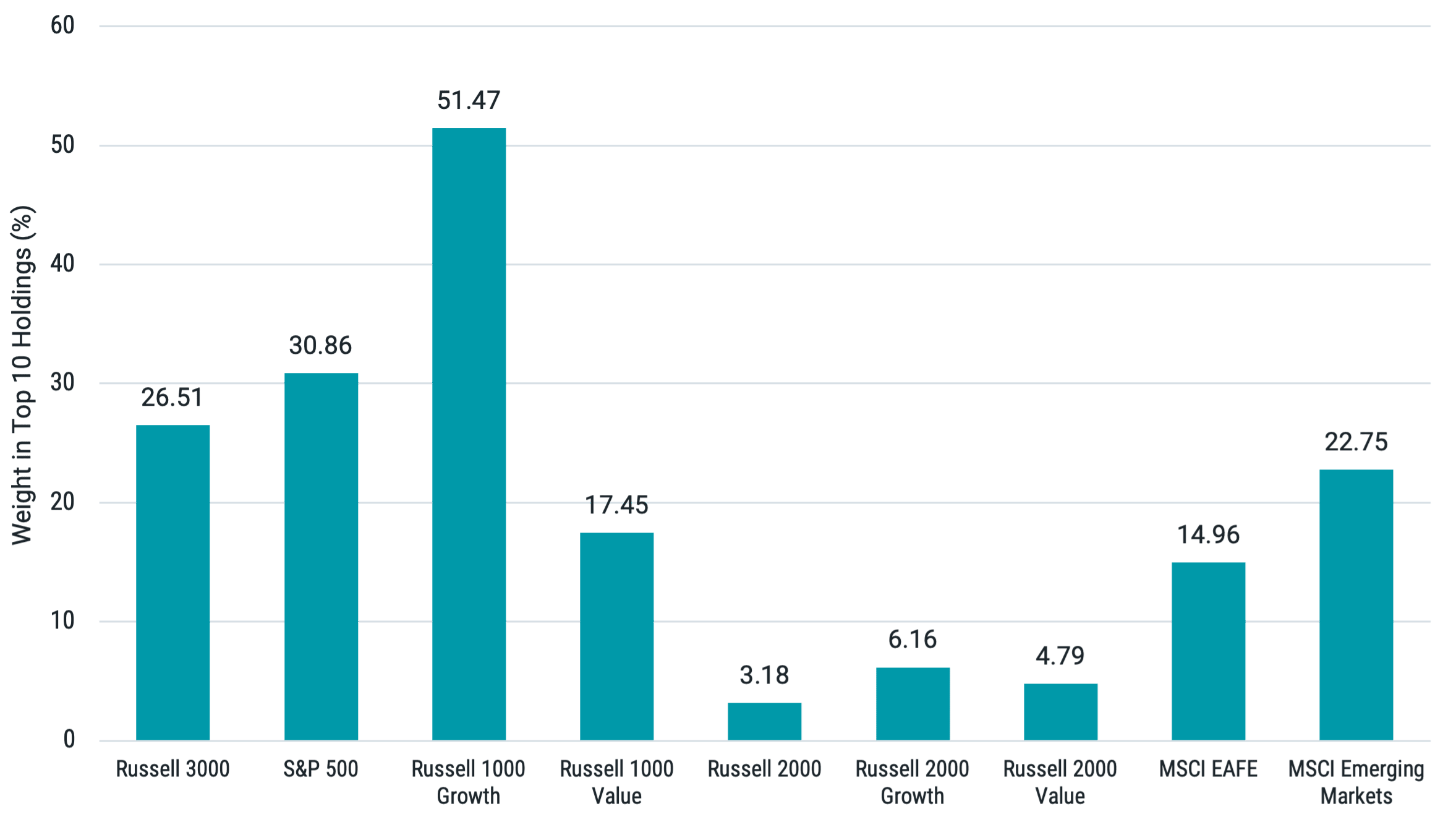
Avantis Investors
The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The option (a) is correct.
Concentration for Google Chrome Extension Download
1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The option (a) is correct. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11.
Concentration Tool APK for Android Download
The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. The option (a) is correct. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ in a 0.150. What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x?
Concentration Legends of Elysium Wikipedia
The option (a) is correct. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. For h2x, ka1=4.5×10−6 and ka2=1.2×10−11. The concentration of x²⁻ in a 0.150.
The Option (A) Is Correct.
What is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x? The concentration of x²⁻ is found to be 2.3×10⁻⁷ m. The concentration of x²⁻ in a 0.150. 1) what is the concentration of x2− in a 0.150 m solution of the diprotic acid h2x?
For H2X, Ka1=4.5×10−6 And Ka2=1.2×10−11.
For h2x, ka1=4.5×10−6 and ka2=1.2×10−11.