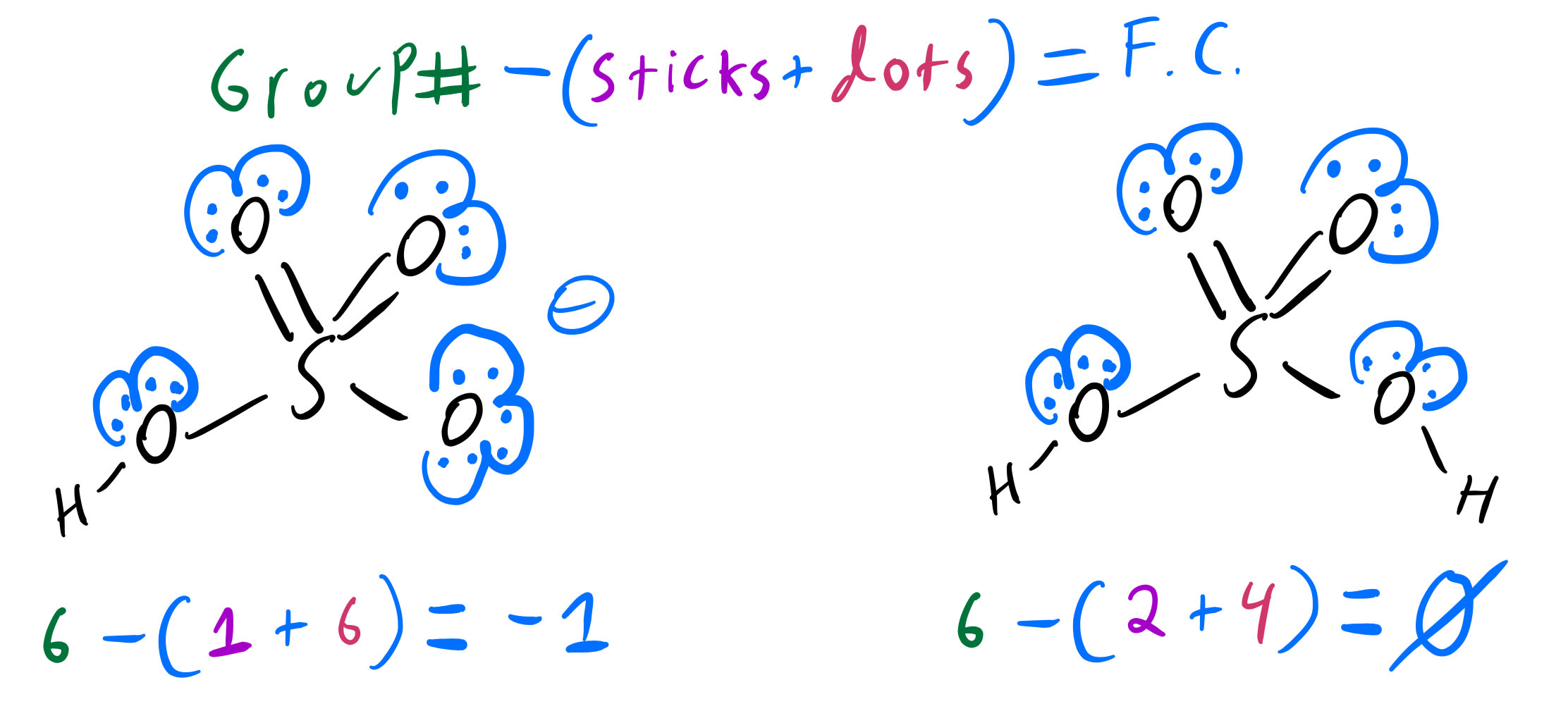
What Is The Conjugate Acid Of H2Po4
What Is The Conjugate Acid Of H2Po4 - The conjugate acid of h2po4 is h3po4. Remove a proton from this, we get,. Simply add or substract a proton from h 3p o4. What are the conjugate acid and base of h 2p o4? Phosphoric acid, h_3po_4, is the parent acid. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen. The conjugate acid of a base, any base, is defined as the base plus a proton. Phosphoric acid is the parent acid, i.e. If it loses a proton, h^+, we conserve both mass and.
The conjugate acid of h2po4 is h3po4. What are the conjugate acid and base of h 2p o4? Phosphoric acid, h_3po_4, is the parent acid. Remove a proton from this, we get,. Phosphoric acid is the parent acid, i.e. Simply add or substract a proton from h 3p o4. If it loses a proton, h^+, we conserve both mass and. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen. The conjugate acid of a base, any base, is defined as the base plus a proton.
Remove a proton from this, we get,. Phosphoric acid is the parent acid, i.e. If it loses a proton, h^+, we conserve both mass and. Phosphoric acid, h_3po_4, is the parent acid. What are the conjugate acid and base of h 2p o4? The conjugate acid of h2po4 is h3po4. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen. Simply add or substract a proton from h 3p o4. The conjugate acid of a base, any base, is defined as the base plus a proton.
Conjugate Acid Of Hpo42 Asking List
Phosphoric acid, h_3po_4, is the parent acid. The conjugate acid of h2po4 is h3po4. Phosphoric acid is the parent acid, i.e. If it loses a proton, h^+, we conserve both mass and. Simply add or substract a proton from h 3p o4.
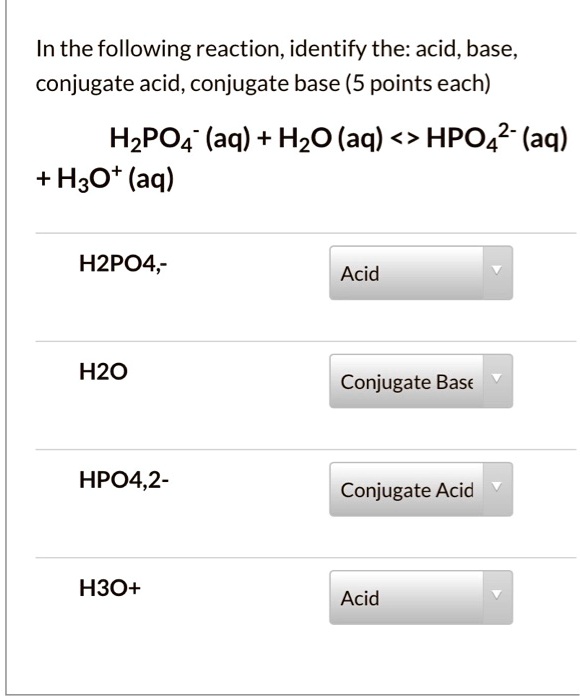
SOLVED In the following reaction, identify the acid, base, conjugate
If it loses a proton, h^+, we conserve both mass and. Remove a proton from this, we get,. What are the conjugate acid and base of h 2p o4? Simply add or substract a proton from h 3p o4. Phosphoric acid, h_3po_4, is the parent acid.
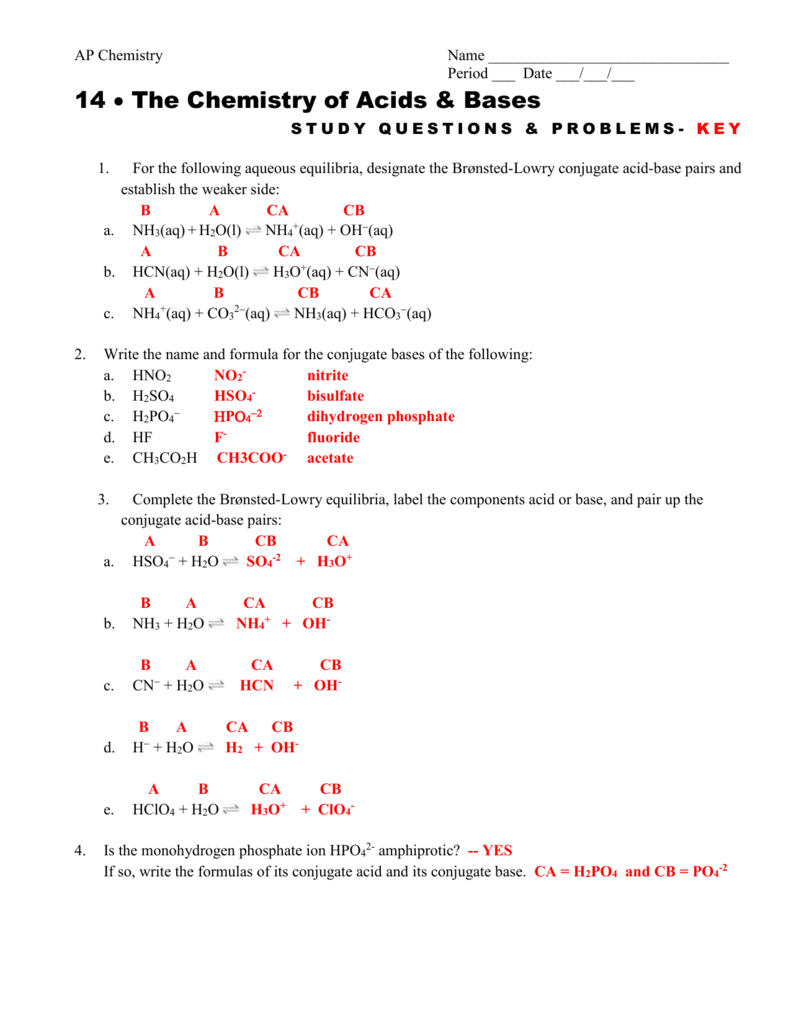
Conjugate Acid Base Pairs Worksheet Answers
What are the conjugate acid and base of h 2p o4? If it loses a proton, h^+, we conserve both mass and. The conjugate acid of h2po4 is h3po4. Simply add or substract a proton from h 3p o4. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen.
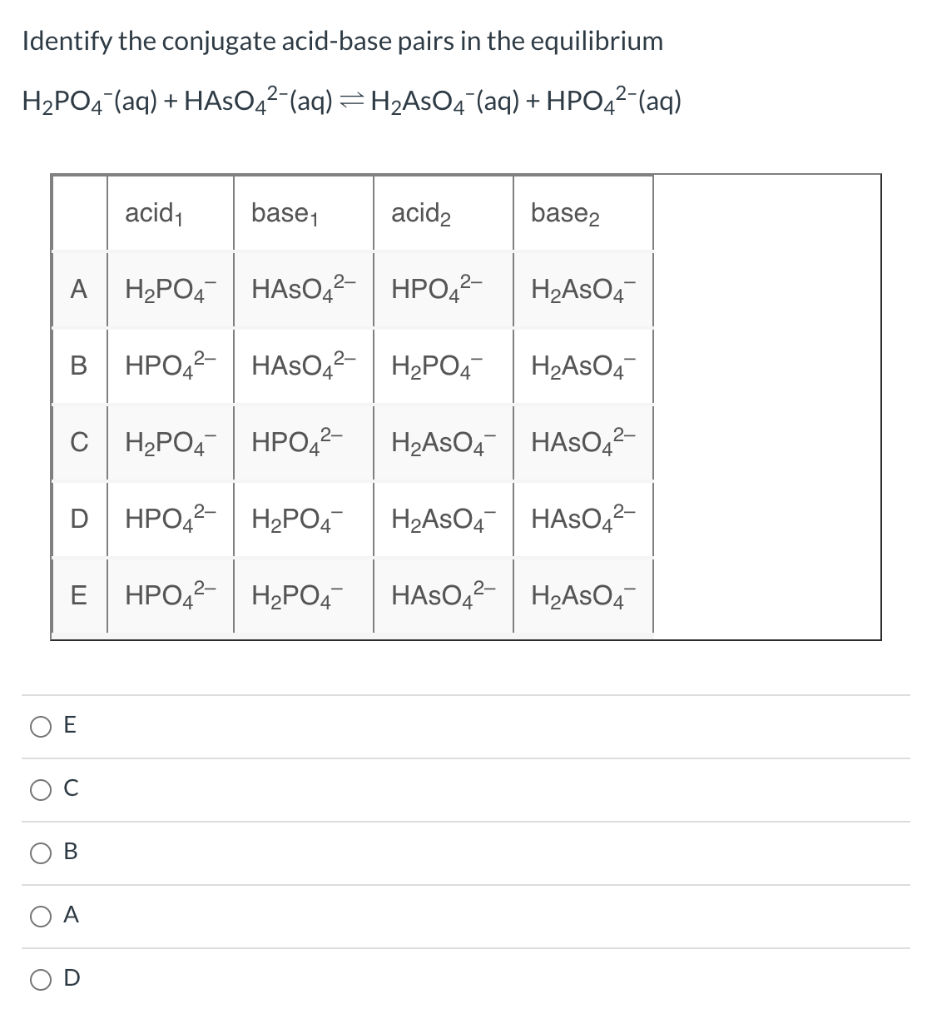
Solved Identify acidbase conjugate pairs. (a) What is the
Phosphoric acid, h_3po_4, is the parent acid. The conjugate acid of h2po4 is h3po4. Remove a proton from this, we get,. The conjugate acid of a base, any base, is defined as the base plus a proton. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen.
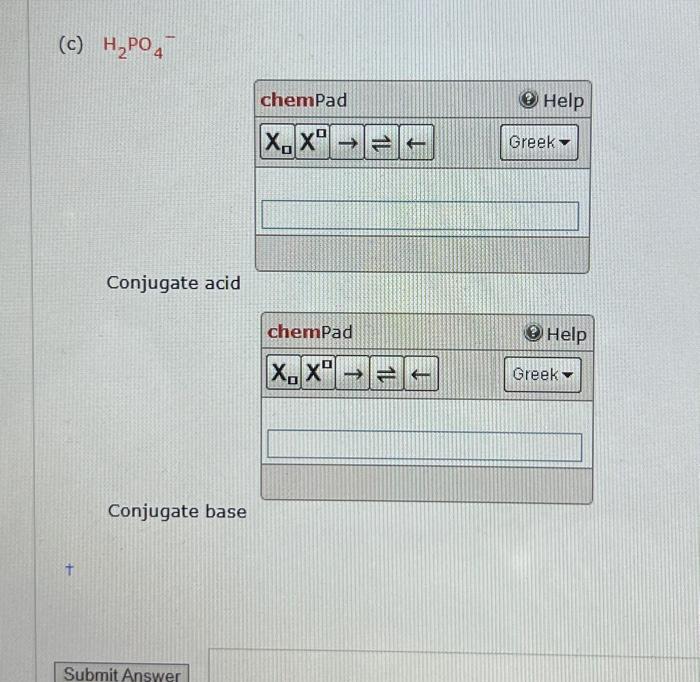
Solved H2PO4− Conjugate acid Conjugate base
The conjugate acid of a base, any base, is defined as the base plus a proton. What are the conjugate acid and base of h 2p o4? Simply add or substract a proton from h 3p o4. Remove a proton from this, we get,. Phosphoric acid is the parent acid, i.e.
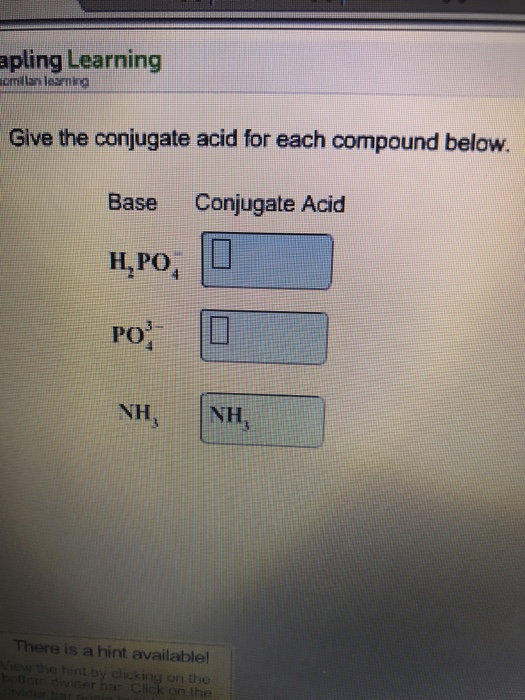
Solved Give the conjugate acid for each compound below.
The conjugate acid of a base, any base, is defined as the base plus a proton. Remove a proton from this, we get,. Simply add or substract a proton from h 3p o4. Phosphoric acid, h_3po_4, is the parent acid. If it loses a proton, h^+, we conserve both mass and.
Conjugate Acid Of H2po4 Asking List
The conjugate acid of a base, any base, is defined as the base plus a proton. Phosphoric acid is the parent acid, i.e. The conjugate acid of h2po4 is h3po4. Phosphoric acid, h_3po_4, is the parent acid. If it loses a proton, h^+, we conserve both mass and.
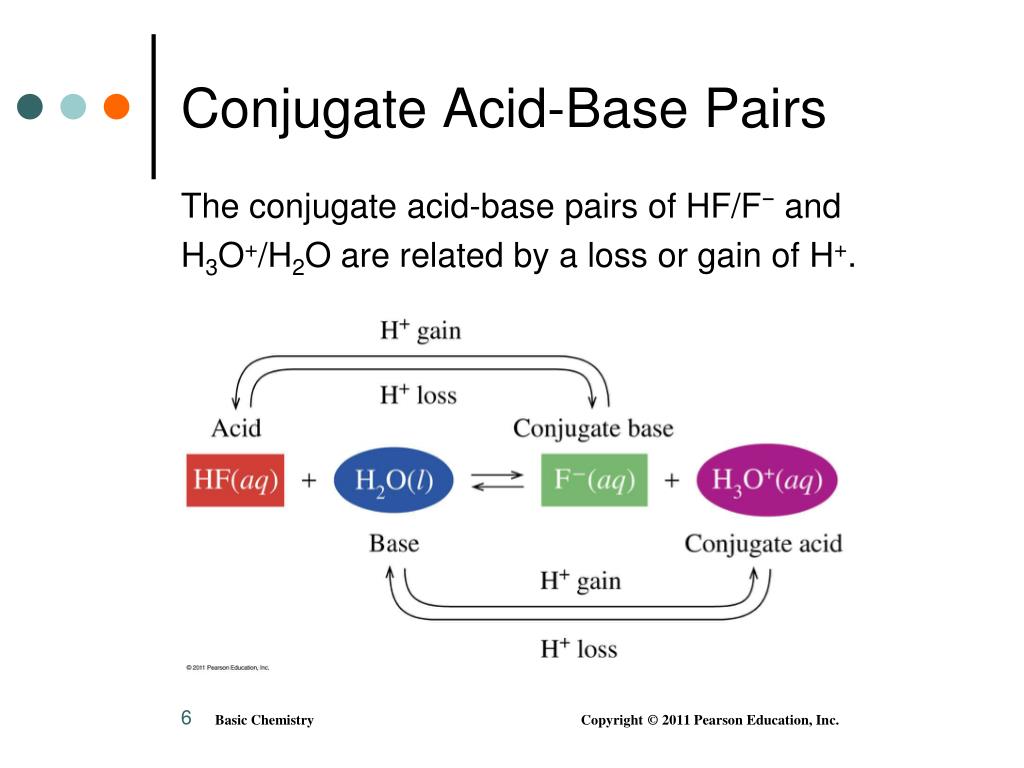
Conjugate Acid Vs Conjugate Base
Remove a proton from this, we get,. The conjugate acid of a base, any base, is defined as the base plus a proton. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen. Simply add or substract a proton from h 3p o4. What are the conjugate acid and base.
SOLVED The pKas of the conjugate acids of OH (conjugate acid H2O) and
The conjugate acid of a base, any base, is defined as the base plus a proton. Phosphoric acid, h_3po_4, is the parent acid. What are the conjugate acid and base of h 2p o4? The conjugate acid of h2po4 is h3po4. If it loses a proton, h^+, we conserve both mass and.
Solved What are the conjugate base and conjugate acid of
What are the conjugate acid and base of h 2p o4? If it loses a proton, h^+, we conserve both mass and. Phosphoric acid is the parent acid, i.e. Phosphoric acid, h_3po_4, is the parent acid. The conjugate acid of a base, any base, is defined as the base plus a proton.
What Are The Conjugate Acid And Base Of H 2P O4?
Remove a proton from this, we get,. If it loses a proton, h^+, we conserve both mass and. The conjugate acid of h2po4 is h3po4. Acids are substances that release hydrogen ions (h+) in aqueous solutions, resulting in an increase in the concentration of hydrogen.
Phosphoric Acid, H_3Po_4, Is The Parent Acid.
Phosphoric acid is the parent acid, i.e. The conjugate acid of a base, any base, is defined as the base plus a proton. Simply add or substract a proton from h 3p o4.