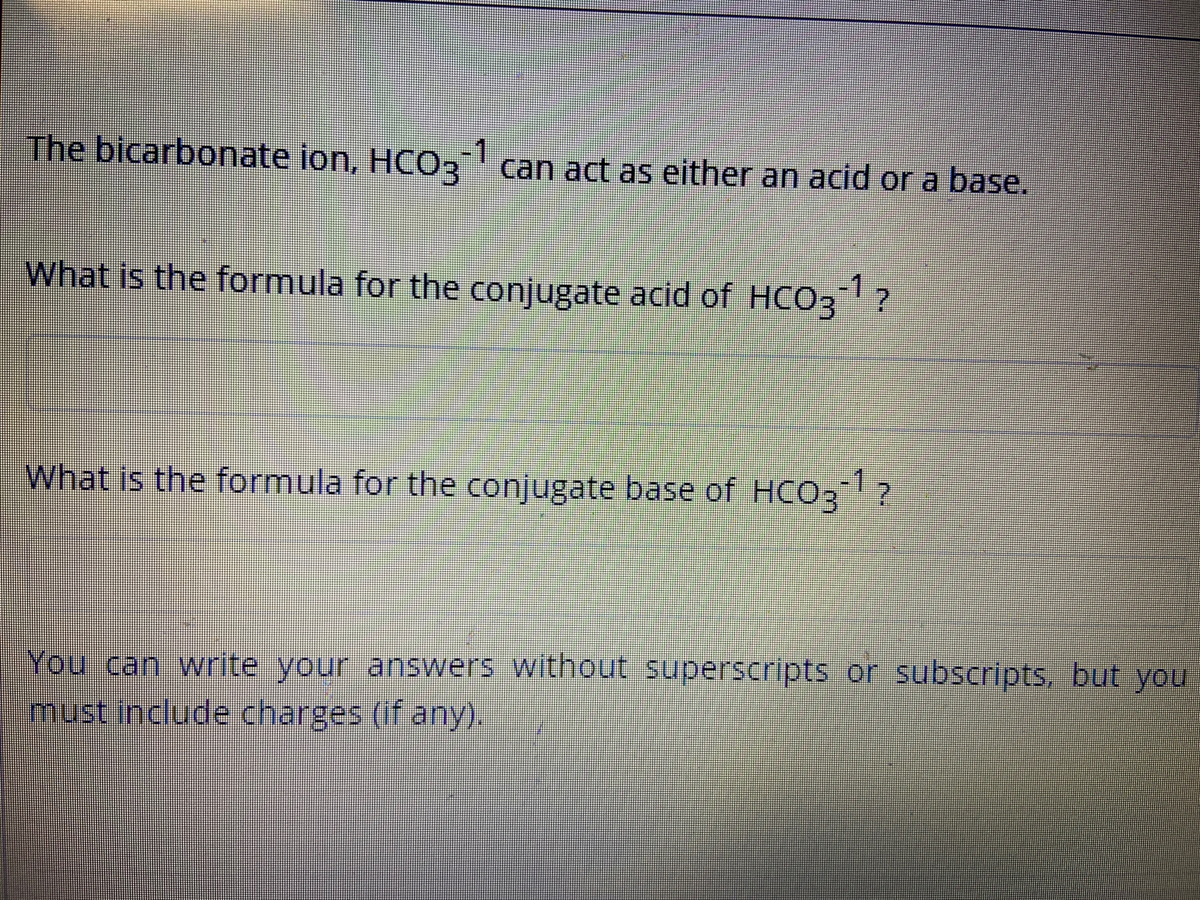
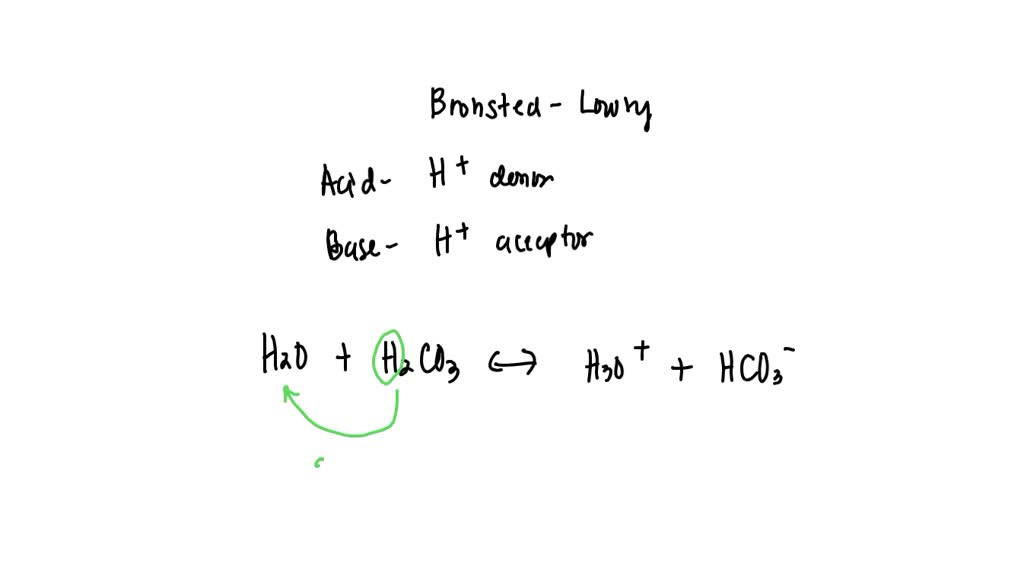What Is The Conjugate Acid Of Hco3
What Is The Conjugate Acid Of Hco3 - Conjugate acids have one extra proton compared to the species that is forming it. Simply add one proton to the formula of the substance to determine the. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. When a proton is added to a base, a conjugate acid is formed. This is formed by adding a proton (h+) to the.
This is formed by adding a proton (h+) to the. Conjugate acids have one extra proton compared to the species that is forming it. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. Simply add one proton to the formula of the substance to determine the. When a proton is added to a base, a conjugate acid is formed.
Simply add one proton to the formula of the substance to determine the. Conjugate acids have one extra proton compared to the species that is forming it. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. This is formed by adding a proton (h+) to the. When a proton is added to a base, a conjugate acid is formed.
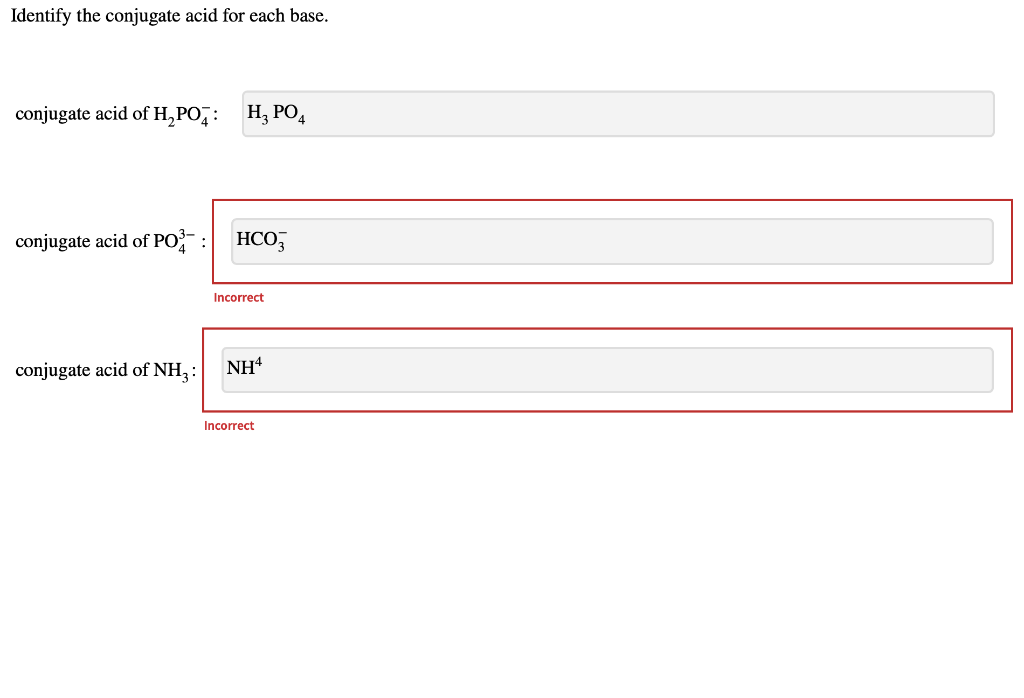
Solved Identify the conjugate acid for each base. conjugate
Simply add one proton to the formula of the substance to determine the. When a proton is added to a base, a conjugate acid is formed. Conjugate acids have one extra proton compared to the species that is forming it. This is formed by adding a proton (h+) to the. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by.
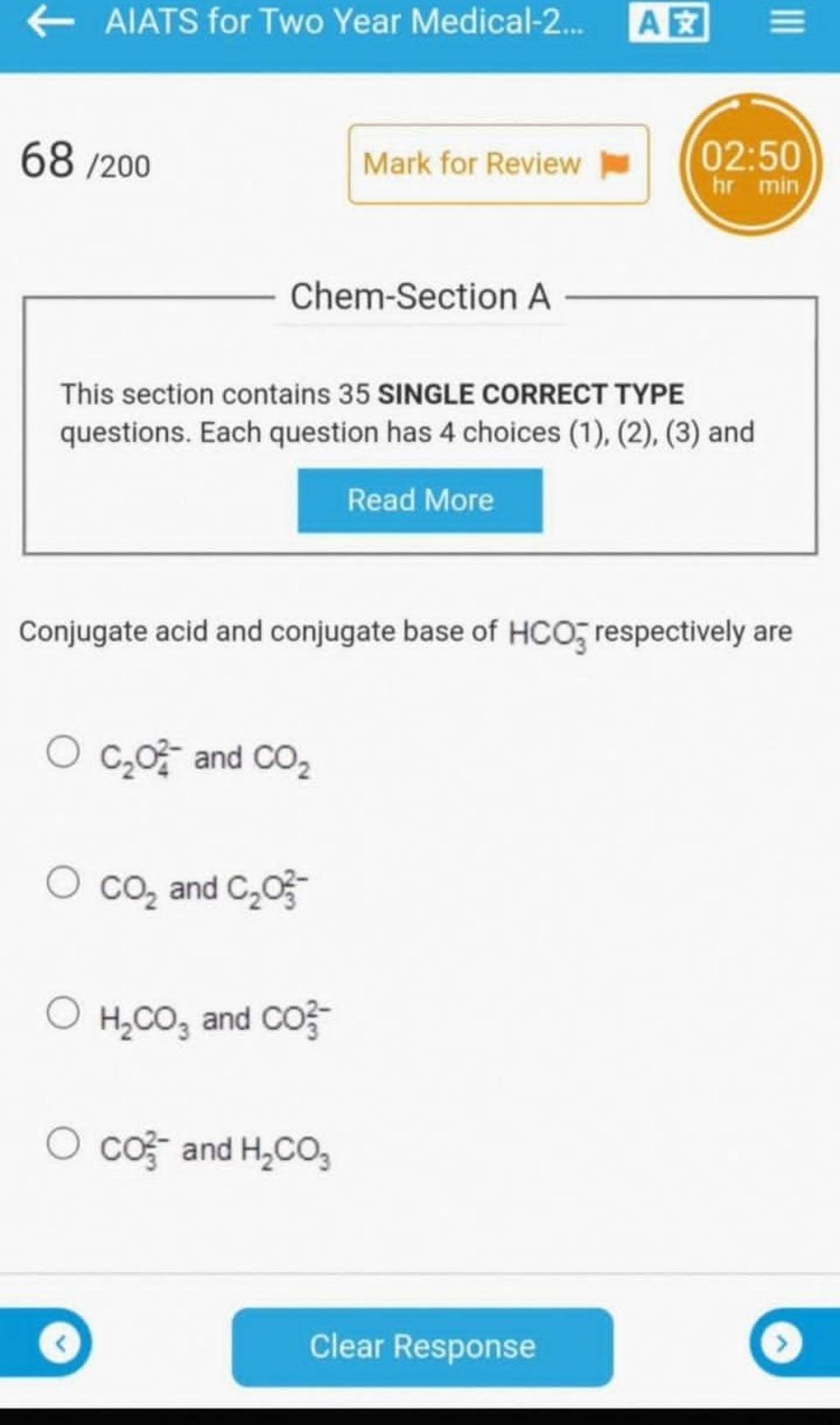
Conjugate acid and conjugate base of HCO3− respectively are Filo
Conjugate acids have one extra proton compared to the species that is forming it. Simply add one proton to the formula of the substance to determine the. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. When a proton is added to a base, a conjugate acid is formed. This is formed.
Conjugate AcidBase Pairs — Overview & Examples Expii
Simply add one proton to the formula of the substance to determine the. Conjugate acids have one extra proton compared to the species that is forming it. When a proton is added to a base, a conjugate acid is formed. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. This is formed.
SOLVED Identify acidbase conjugate pairs. Close Problem (a) What is the conjugate acid of F
This is formed by adding a proton (h+) to the. When a proton is added to a base, a conjugate acid is formed. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. Simply add one proton to the formula of the substance to determine the. Conjugate acids have one extra proton compared.
How To Draw A Conjugate Acid
This is formed by adding a proton (h+) to the. When a proton is added to a base, a conjugate acid is formed. Simply add one proton to the formula of the substance to determine the. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. Conjugate acids have one extra proton compared.
Solved Exercises What Is The Conjugate Acid Of HCO3?, 47 OFF
This is formed by adding a proton (h+) to the. Conjugate acids have one extra proton compared to the species that is forming it. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. Simply add one proton to the formula of the substance to determine the. When a proton is added to.
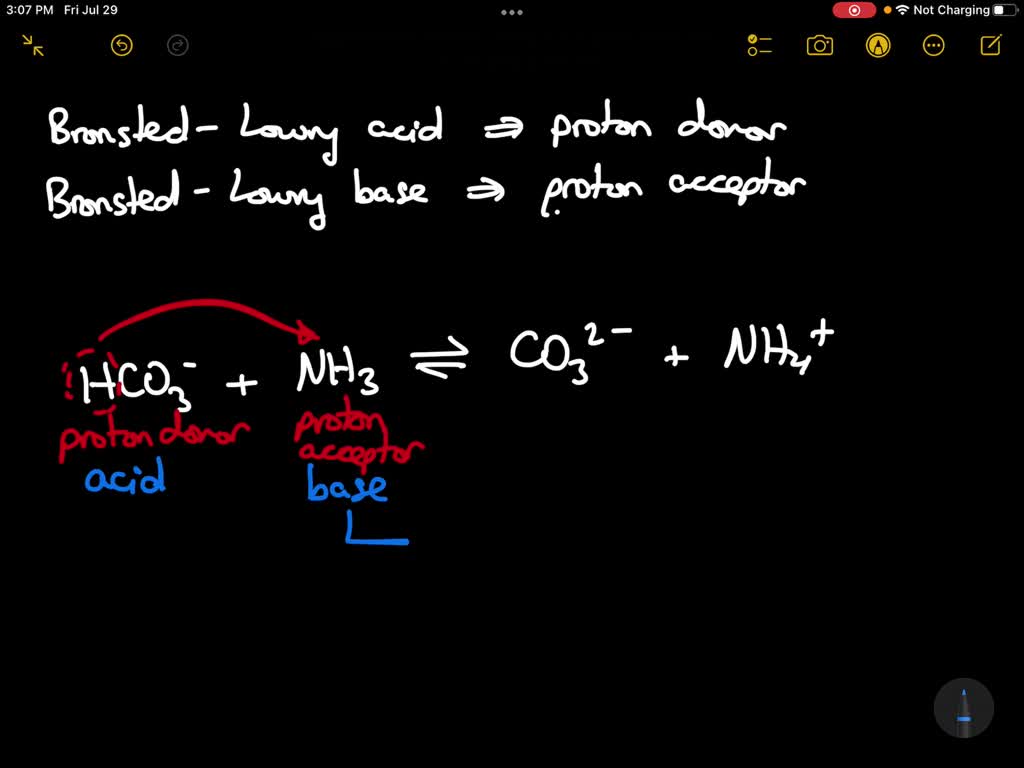
SOLVED In the following reaction, what is the acid, the base, the conjugate acid, the conjugate
This is formed by adding a proton (h+) to the. When a proton is added to a base, a conjugate acid is formed. Simply add one proton to the formula of the substance to determine the. Conjugate acids have one extra proton compared to the species that is forming it. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by.
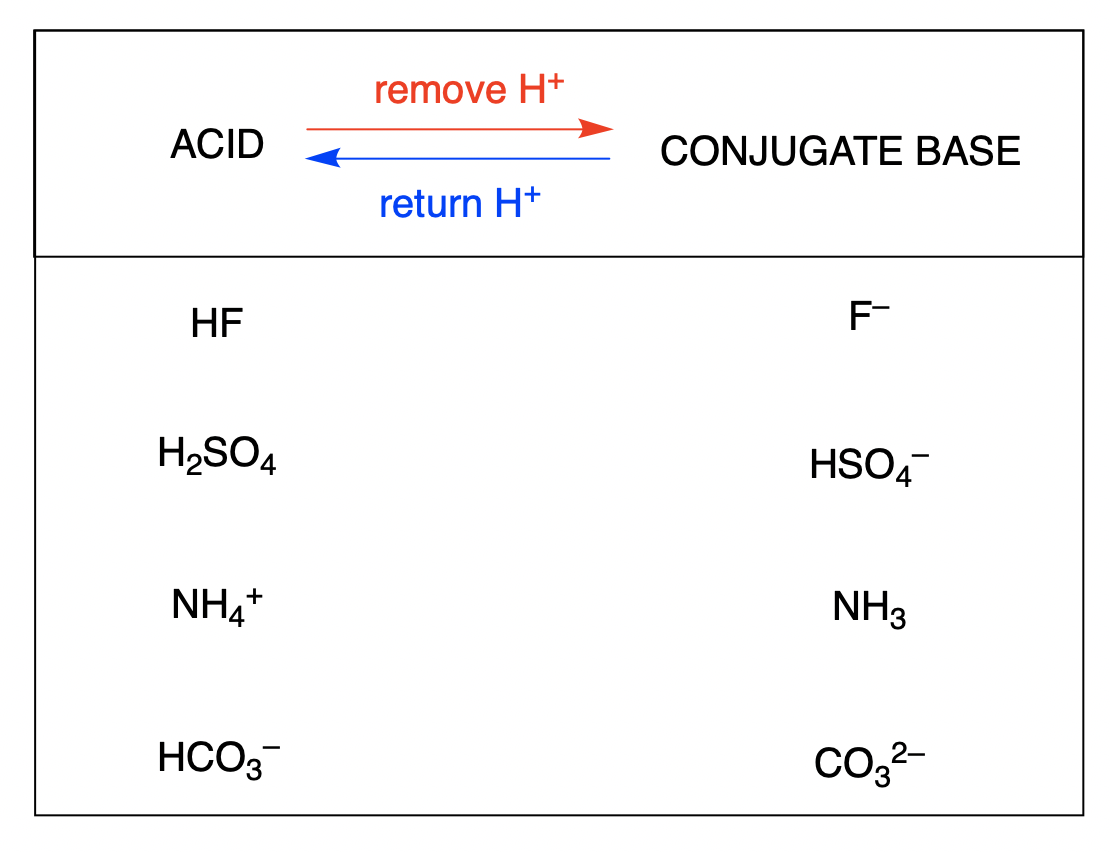
Conjugate Acids And Bases Acid Base Equilibria
The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. Simply add one proton to the formula of the substance to determine the. Conjugate acids have one extra proton compared to the species that is forming it. When a proton is added to a base, a conjugate acid is formed. This is formed.
SOLVED For the reaction below, identify the acid, the base, the conjugate acid, and the
When a proton is added to a base, a conjugate acid is formed. Conjugate acids have one extra proton compared to the species that is forming it. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. This is formed by adding a proton (h+) to the. Simply add one proton to the.
Answered Identify the conjugate acid for each… bartleby
Simply add one proton to the formula of the substance to determine the. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. This is formed by adding a proton (h+) to the. When a proton is added to a base, a conjugate acid is formed. Conjugate acids have one extra proton compared.
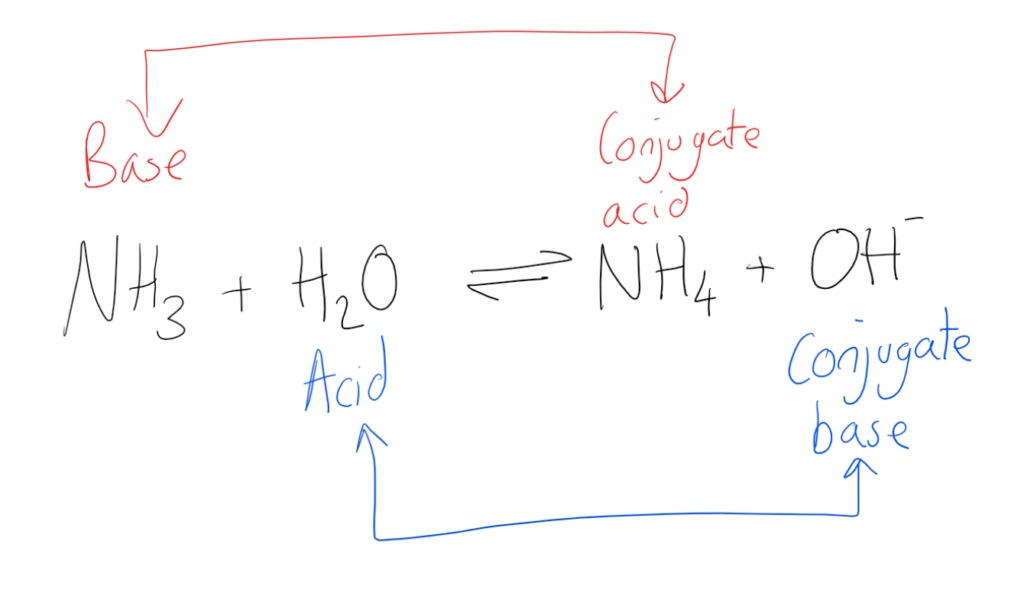
When A Proton Is Added To A Base, A Conjugate Acid Is Formed.
Conjugate acids have one extra proton compared to the species that is forming it. Simply add one proton to the formula of the substance to determine the. The conjugate acid of hco3⁻ (bicarbonate ion) is formed by adding one proton (h⁺) to the ion. This is formed by adding a proton (h+) to the.