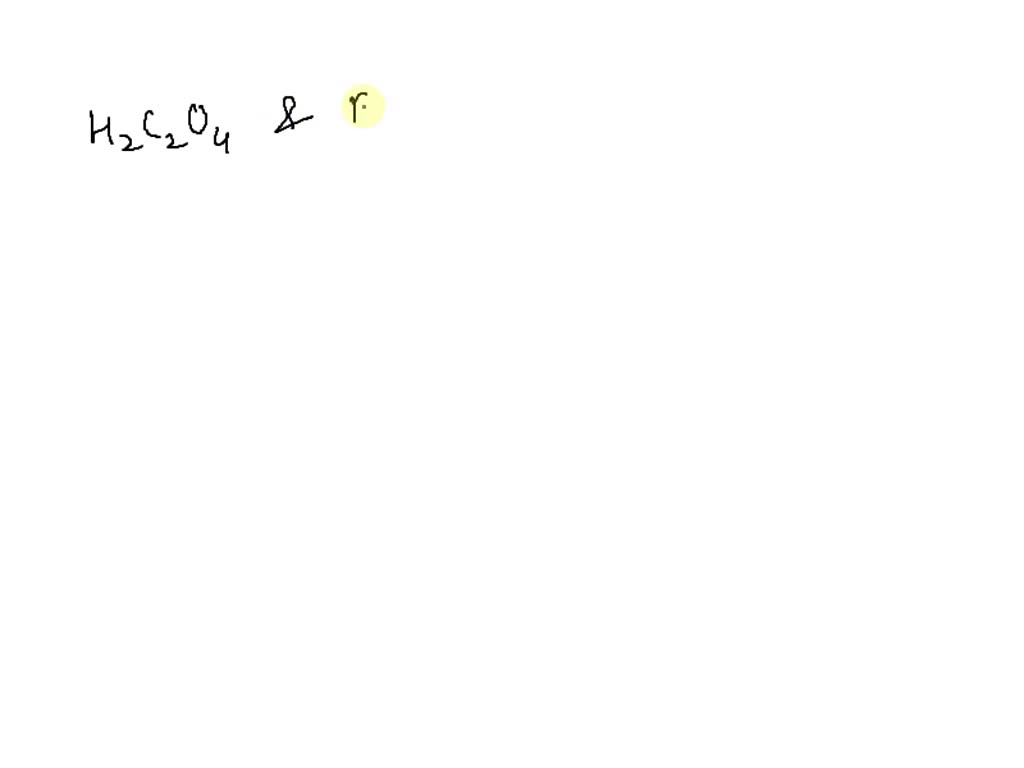What Is The Conjugate Base Of Hco3
What Is The Conjugate Base Of Hco3 - For each case give the corresponding. Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. When an acid, like bicarbonate ion, donates a proton, it transforms into its.
When an acid, like bicarbonate ion, donates a proton, it transforms into its. For each case give the corresponding. Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
When an acid, like bicarbonate ion, donates a proton, it transforms into its. For each case give the corresponding. Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
SOLVED What is the conjugate base for HCH3CO2 and the conjugate base
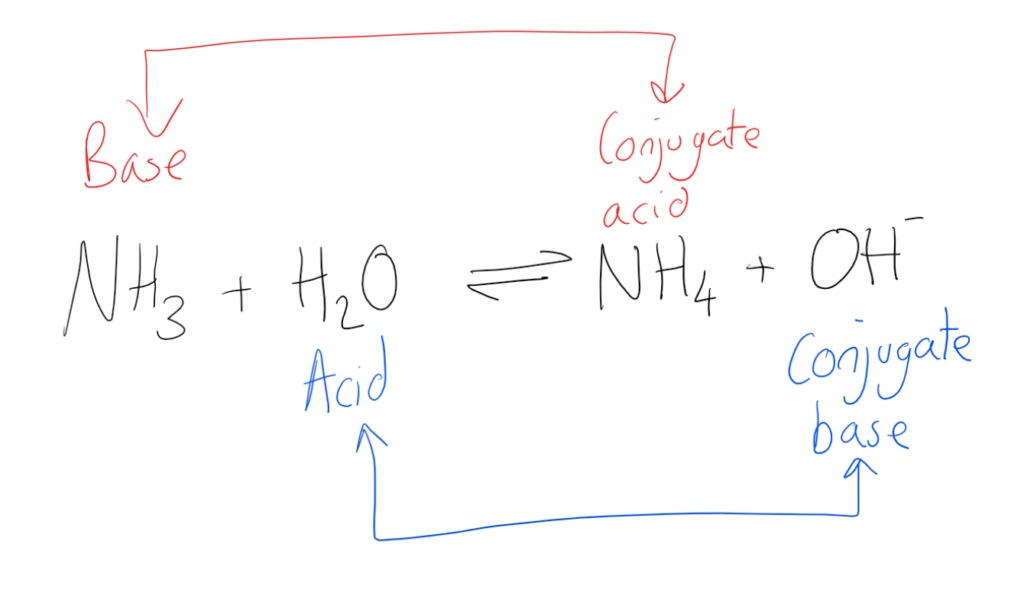
Nh4+ is the conjugate acid to the base nh3, because. When an acid, like bicarbonate ion, donates a proton, it transforms into its. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. For each case give the corresponding.
Solved Give The Conjugate Base For Each Compound Below. A...
For each case give the corresponding. Nh4+ is the conjugate acid to the base nh3, because. When an acid, like bicarbonate ion, donates a proton, it transforms into its. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
Conjugate AcidBase Pairs — Overview & Examples Expii
Nh4+ is the conjugate acid to the base nh3, because. When an acid, like bicarbonate ion, donates a proton, it transforms into its. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. For each case give the corresponding.
Conjugate Acids And Bases Acid Base Equilibria
For each case give the corresponding. When an acid, like bicarbonate ion, donates a proton, it transforms into its. Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
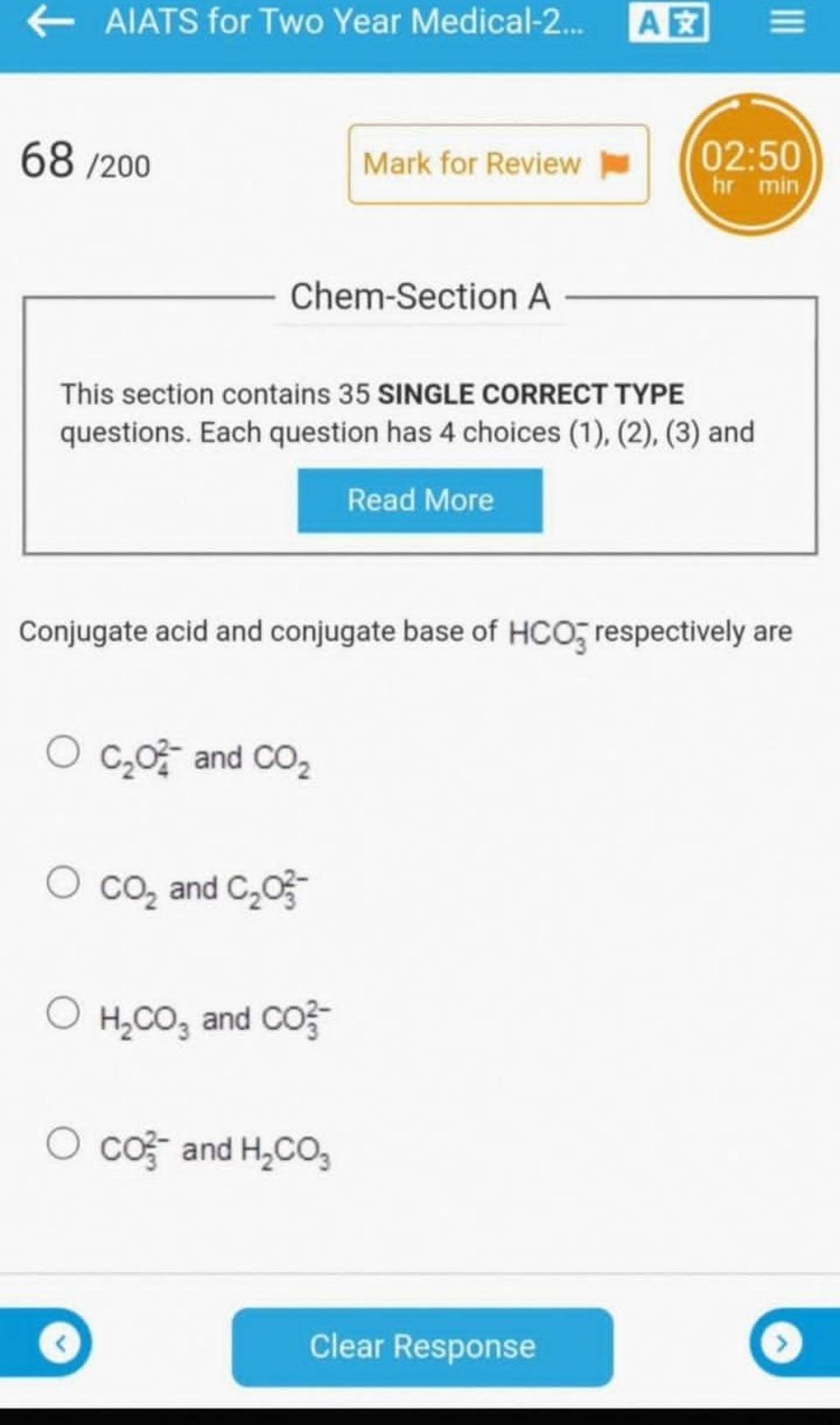
Conjugate acid and conjugate base of HCO3− respectively are Filo
Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. When an acid, like bicarbonate ion, donates a proton, it transforms into its. For each case give the corresponding.
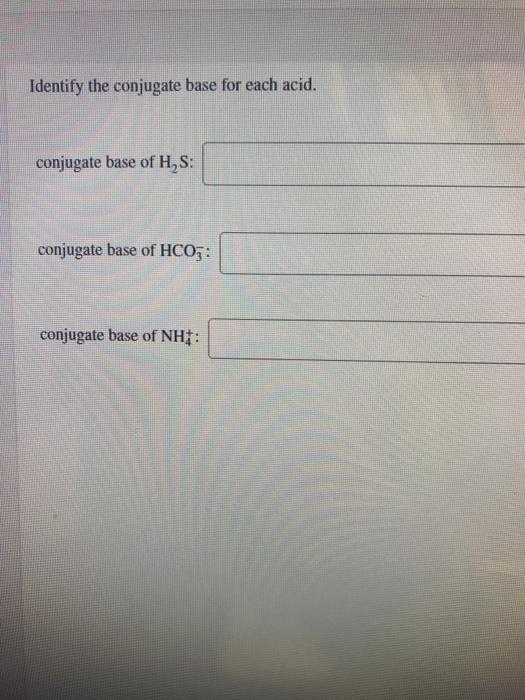
Solved Identify the conjugate base for each acid. conjugate
When an acid, like bicarbonate ion, donates a proton, it transforms into its. For each case give the corresponding. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. Nh4+ is the conjugate acid to the base nh3, because.
Solved The conjugate base of H2CO3 is H2CO3 HCO3 CO32 O
Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. When an acid, like bicarbonate ion, donates a proton, it transforms into its. Nh4+ is the conjugate acid to the base nh3, because. For each case give the corresponding.
Solved Draw the conjugate base of the following Acids
When an acid, like bicarbonate ion, donates a proton, it transforms into its. For each case give the corresponding. Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
Solved Identify the conjugate base for each acid. conjugate
For each case give the corresponding. When an acid, like bicarbonate ion, donates a proton, it transforms into its. Nh4+ is the conjugate acid to the base nh3, because. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
Conjugate Acids And Conjugate Bases Are The Acids And Bases That Lose Or Gain Protons.
When an acid, like bicarbonate ion, donates a proton, it transforms into its. Nh4+ is the conjugate acid to the base nh3, because. For each case give the corresponding.