What Is The Difference Between Electron Geometry And Molecular Geometry
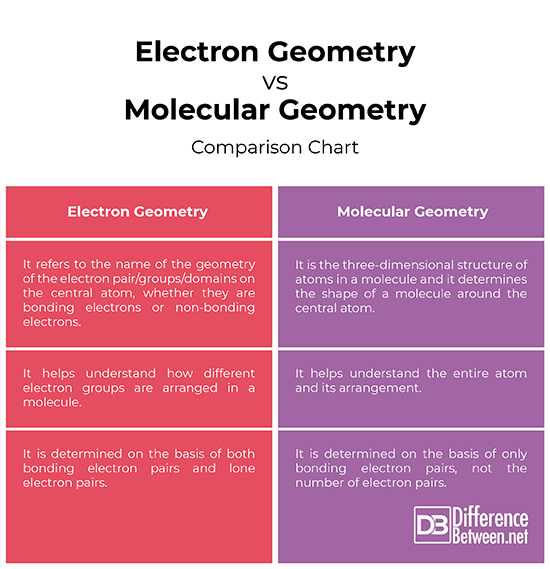
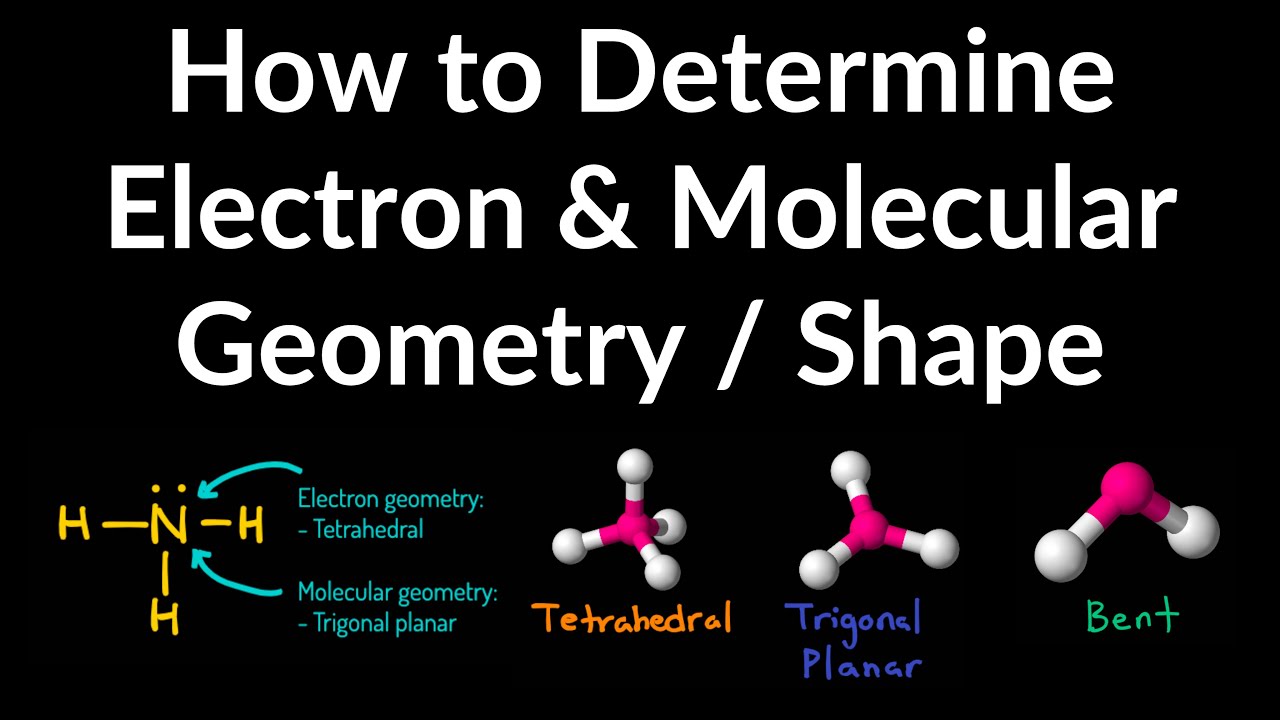
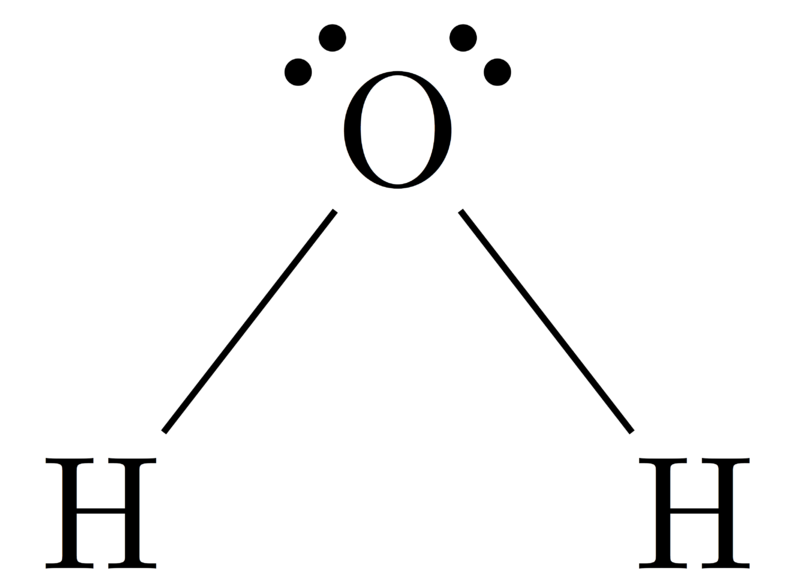
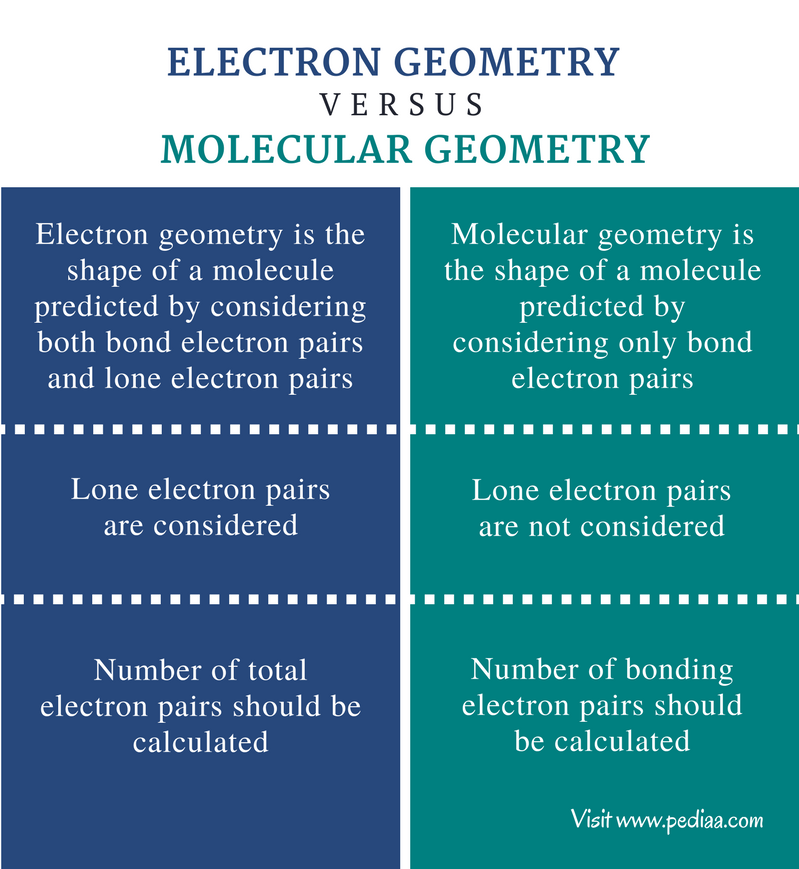
What Is The Difference Between Electron Geometry And Molecular Geometry - Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,.
One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,.
One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,.
Electron vs Molecular Geometry Difference and Comparison
One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the..
Electron Geometry vs. Molecular Geometry — What’s the Difference?
Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry refers to.
Print Difference Between Print
Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,. Electron geometry refers to.
Electron geometry molecular geometry chart hybridization luvshery
Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the..
Difference Between Electron Geometry and Molecular Geometry
Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the..
Difference Between Electron Geometry and Molecular Geometry
Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons..
Difference Between Electron Geometry and Molecular Geometry
One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the..
What is the difference between electron geometry and molecular geometry
Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry refers to.
Difference Between Electron Geometry and Molecular Geometry
Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. One of the.
Difference Between Electron Geometry and Molecular Geometry
Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the. Electron geometry differs from molecular geometry in that it encompasses all electron groups surrounding the central atom,. Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. One of the.
Electron Geometry Differs From Molecular Geometry In That It Encompasses All Electron Groups Surrounding The Central Atom,.
Electron geometry refers to the arrangement of electron groups around the central atom, including both bonding and lone pairs of electrons. One of the key differences between electron geometry and molecular geometry is that electron geometry is determined solely by the. Electron geometry refers to the spatial arrangement of electron pairs around the central atom, while molecular geometry describes the.