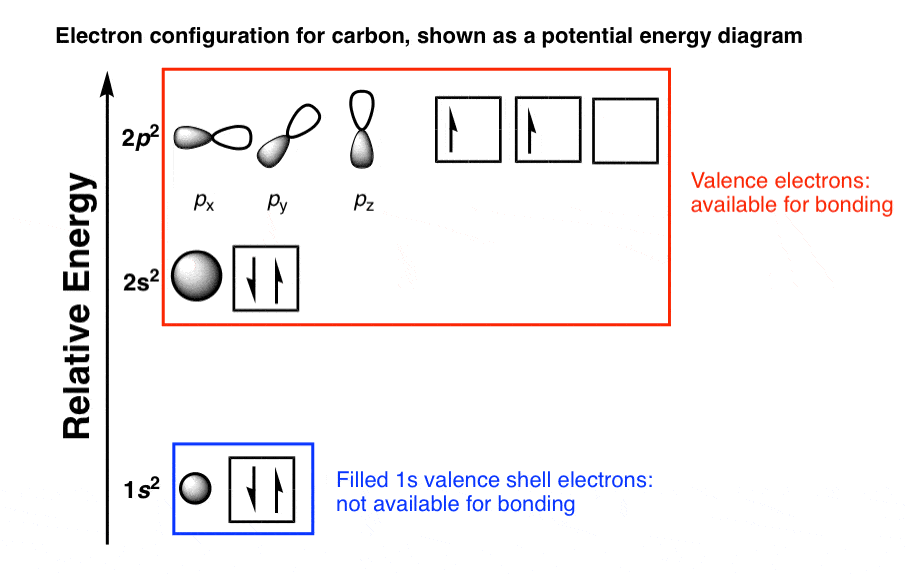
What Is The Electron Configuration Of Co2
What Is The Electron Configuration Of Co2 - We will remove the 2 electrons. What is the electronic configuration of co atom and a co2+ ion ?? The electron configuration of the co2 ion is 1s² 2s². Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. This ion has 18 electrons, two fewer than the neutral co. The electron configuration of the co2 ion can be determined. To turn co into co2+ ion, we need to remove 2 electrons. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. The electron configuration of the co2+ ion is 1s2 2s2 2p6.
Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. The electron configuration of the co2 ion is 1s² 2s². We will remove the 2 electrons. The electron configuration of the co2 ion can be determined. What is the electronic configuration of co atom and a co2+ ion ?? Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. This ion has 18 electrons, two fewer than the neutral co. The electron configuration of the co2+ ion is 1s2 2s2 2p6. To turn co into co2+ ion, we need to remove 2 electrons.
We will remove the 2 electrons. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. The electron configuration of the co2+ ion is 1s2 2s2 2p6. What is the electronic configuration of co atom and a co2+ ion ?? Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. The electron configuration of the co2 ion is 1s² 2s². To turn co into co2+ ion, we need to remove 2 electrons. This ion has 18 electrons, two fewer than the neutral co. The electron configuration of the co2 ion can be determined.
Carbon electron configuration aufbau principle tatacycle
Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. The electron configuration of the co2+ ion is 1s2 2s2 2p6. To turn co into co2+ ion, we need to remove 2 electrons. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. This ion has 18 electrons, two fewer than the neutral co.
Carbon electron configuration Stock Image C029/5022 Science Photo Library
What is the electronic configuration of co atom and a co2+ ion ?? Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. The electron configuration of the co2 ion can be determined. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. This ion has 18 electrons, two fewer than the neutral co.
Carbon Electron Configuration Diagram
The electron configuration of the co2+ ion is 1s2 2s2 2p6. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. The electron configuration of the co2 ion is 1s² 2s². Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. To turn co into co2+ ion, we need to remove 2 electrons.
SOLUTION Co2 part 3 electron configuration Studypool
Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. We will remove the 2 electrons. To turn co into co2+ ion, we need to remove 2 electrons. The electron configuration of the co2 ion is 1s² 2s². The electron configuration of the co2 ion can be determined.
SOLUTION Co2 part 3 electron configuration Studypool
Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. This ion has 18 electrons, two fewer than the neutral co. The electron configuration of the co2 ion is 1s² 2s². The electron configuration of the co2+ ion is 1s2 2s2 2p6. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co.
SOLUTION Co2 part 3 electron configuration Studypool
This ion has 18 electrons, two fewer than the neutral co. The electron configuration of the co2+ ion is 1s2 2s2 2p6. We will remove the 2 electrons. To turn co into co2+ ion, we need to remove 2 electrons. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co.
Electron Configuration for Cobalt (Co and Co2+, Co3+)
Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. We will remove the 2 electrons. What is the electronic configuration of co atom and a co2+ ion ?? The electron configuration of the co2 ion can be determined. The electron configuration of the co2 ion is 1s² 2s².
Electron Configuration Electron Shell Valence Electron Carbon, PNG, 800x800px, Electron
Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. The electron configuration of the co2+ ion is 1s2 2s2 2p6. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. The electron configuration of the co2 ion can be determined. To turn co into co2+ ion, we need to remove 2 electrons.
Carbon Electron Configuration Diagram
The electron configuration of the co2 ion is 1s² 2s². We will remove the 2 electrons. What is the electronic configuration of co atom and a co2+ ion ?? This ion has 18 electrons, two fewer than the neutral co. The electron configuration of the co2+ ion is 1s2 2s2 2p6.
SOLUTION Co2 part 3 electron configuration Studypool
Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. The electron configuration of the co2 ion can be determined. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. To turn co into co2+ ion, we need to remove 2 electrons. The electron configuration of the co2+ ion is 1s2 2s2 2p6.
What Is The Electronic Configuration Of Co Atom And A Co2+ Ion ??
Co2+ would most likely be 1s22s22p63s23p64s03d7, and co. The electron configuration of the co2 ion can be determined. This ion has 18 electrons, two fewer than the neutral co. We will remove the 2 electrons.
The Electron Configuration Of The Co2+ Ion Is 1S2 2S2 2P6.
The electron configuration of the co2 ion is 1s² 2s². To turn co into co2+ ion, we need to remove 2 electrons. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2.