What Is The Electron Configuration Of The Calcium Ion
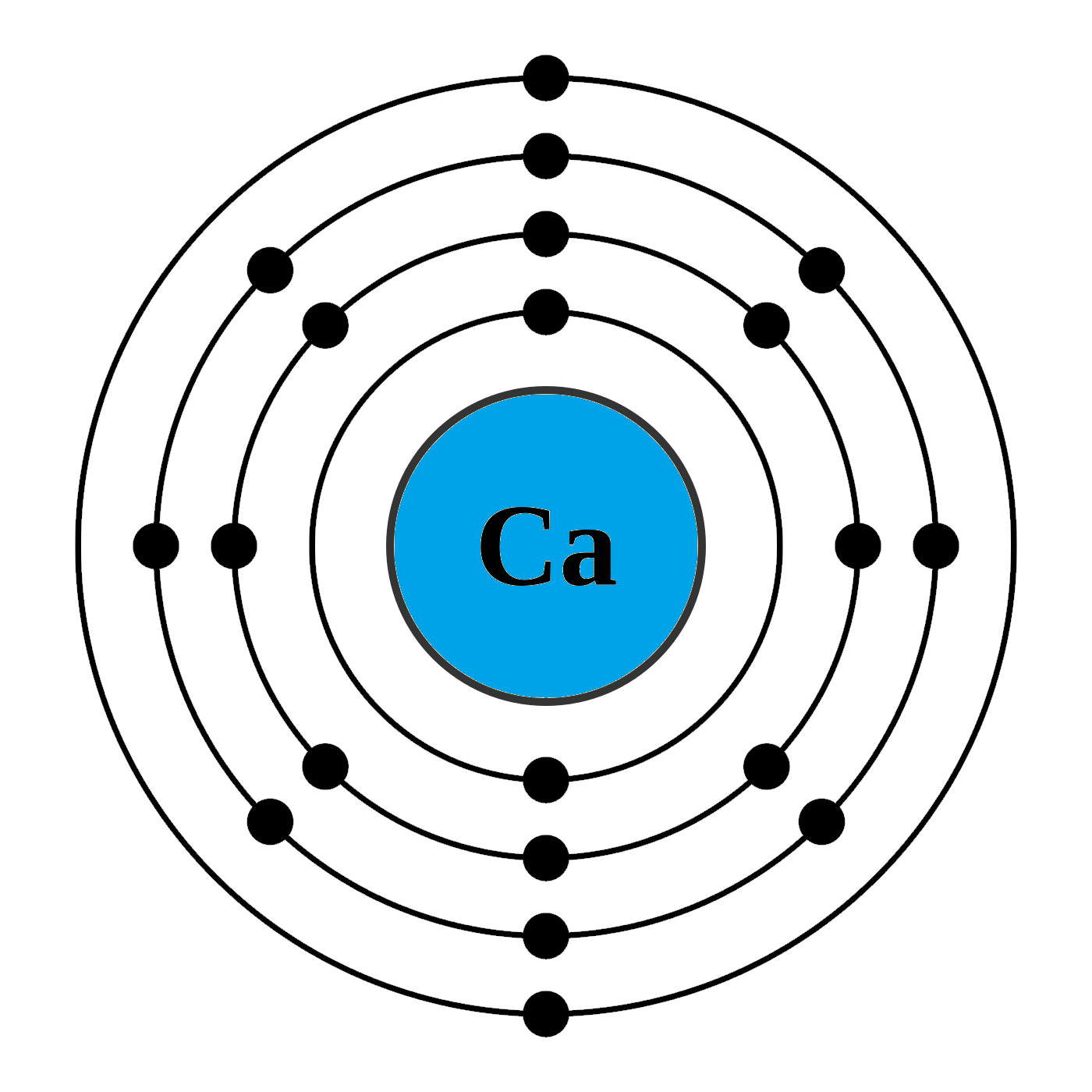
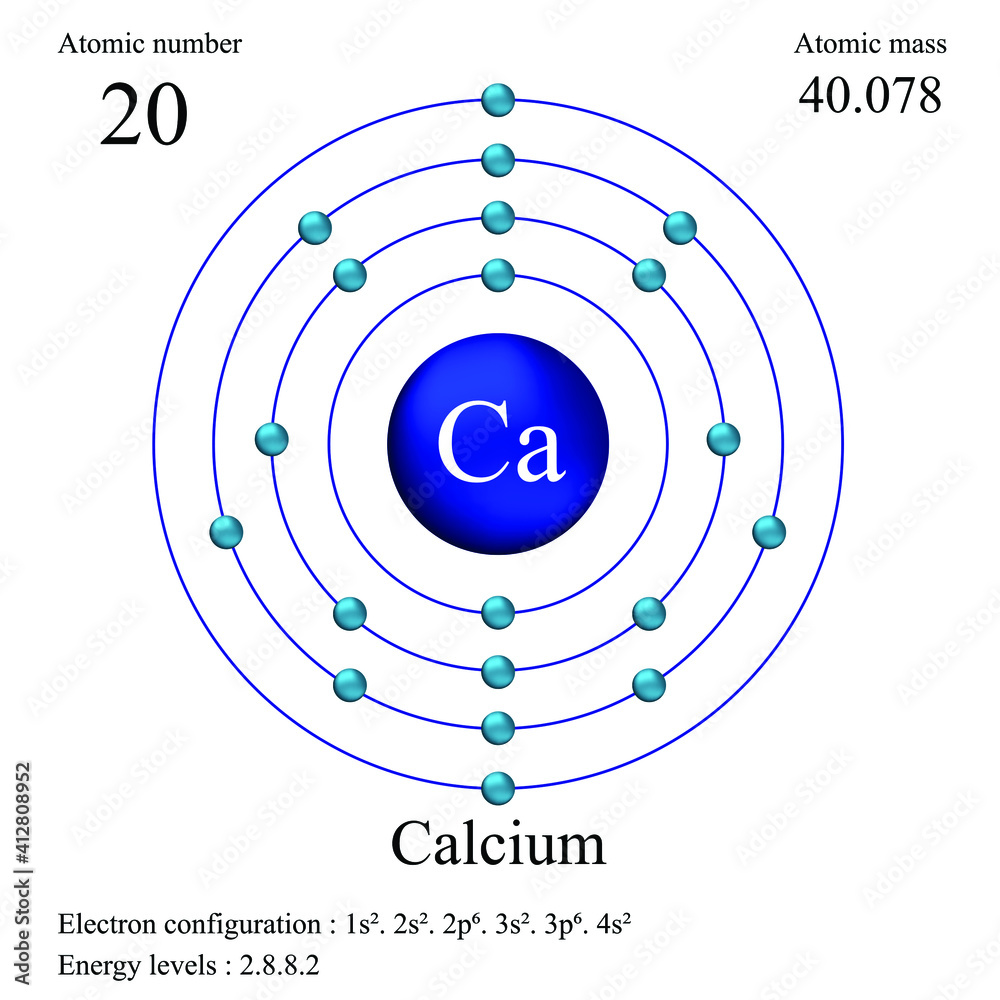
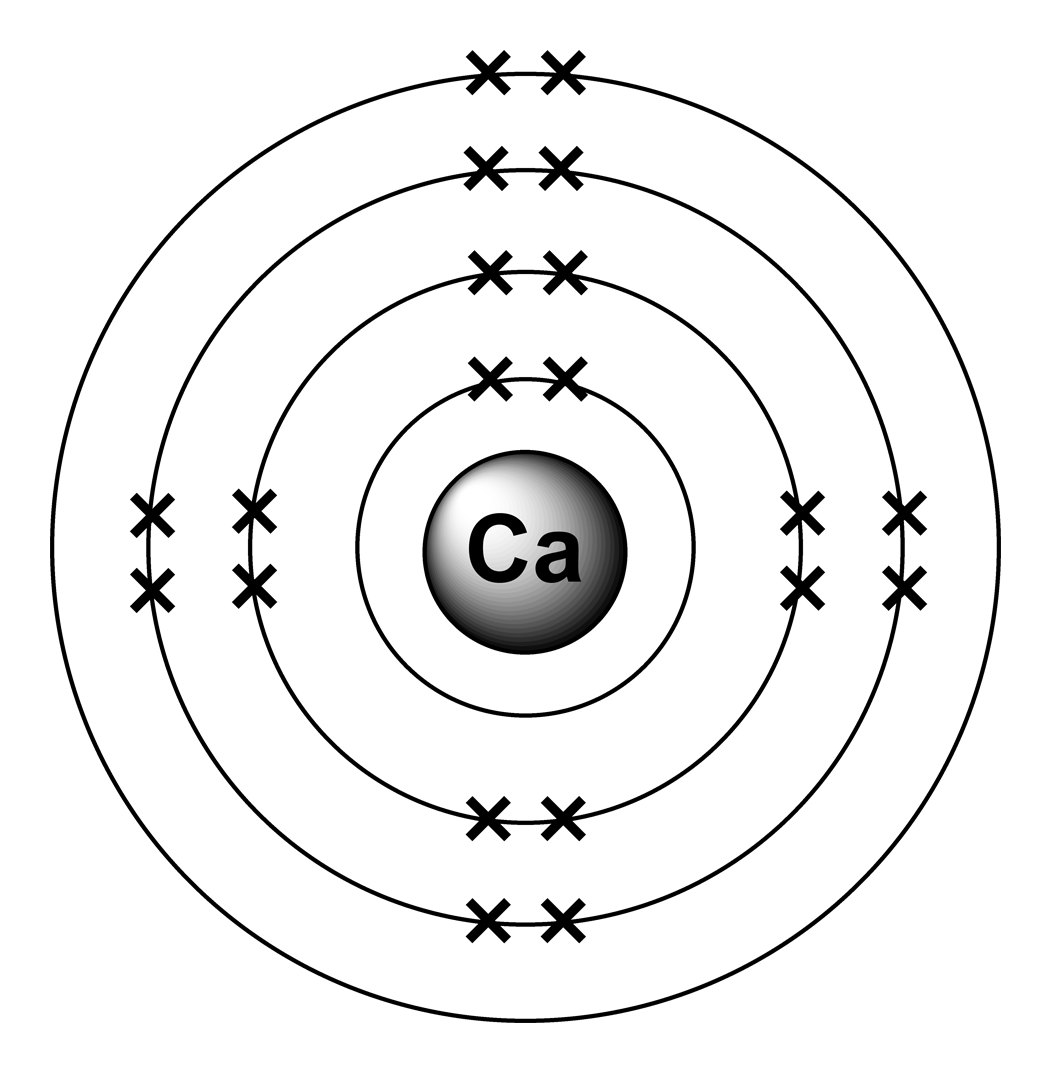
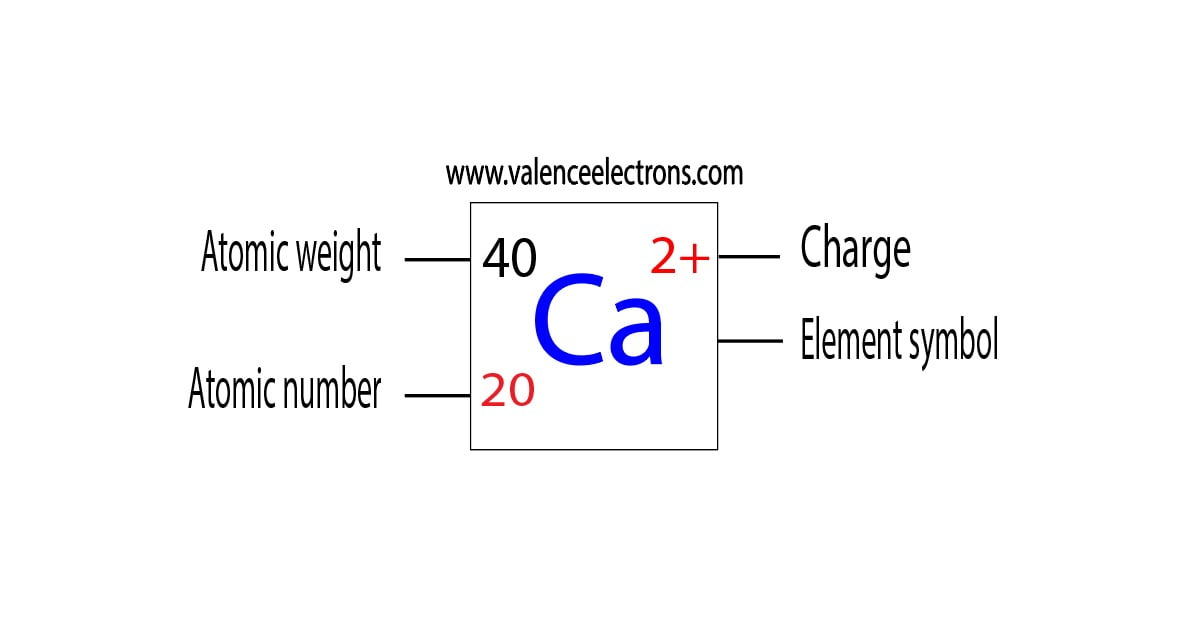
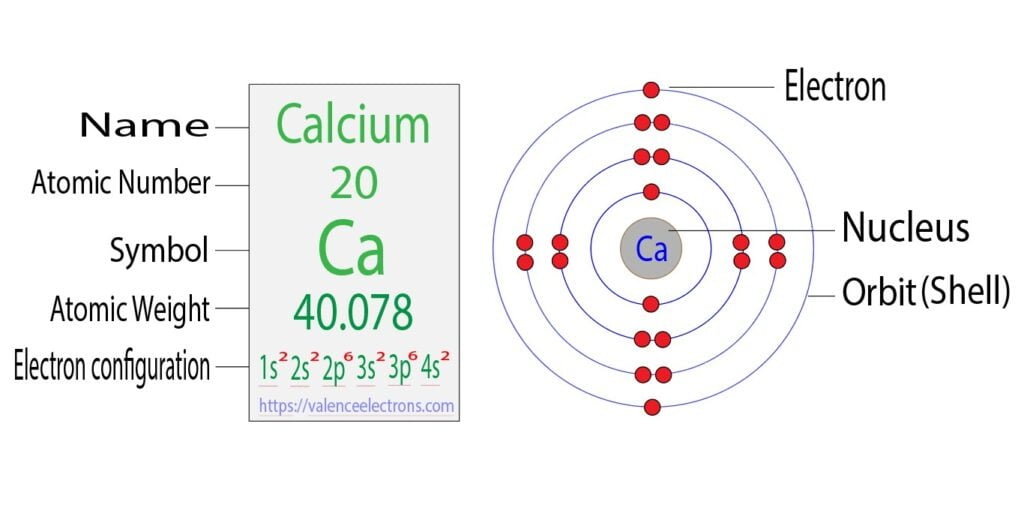
What Is The Electron Configuration Of The Calcium Ion - Calcium has an atomic number of 20. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. 1s^2 2s^2 2p^6 3s^2 3p^6. The calcium ion (ca 2+ ),. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Simply use this information to obtain its electronic configuration. Let's determine the electron configuration of a calcium ion,. The electron configuration of a #ca^(2+)# ion. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). By removing the two outermost electrons from.
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. Let's determine the electron configuration of a calcium ion,. Simply use this information to obtain its electronic configuration. Calcium has an atomic number of 20. The calcium ion (ca 2+ ),. The electron configuration of the calcium ion (ca^2+) is: The electron configuration of a #ca^(2+)# ion. By removing the two outermost electrons from. 1s^2 2s^2 2p^6 3s^2 3p^6.
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). First, we need to know the electron configuration of a neutral. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. Let's determine the electron configuration of a calcium ion,. Simply use this information to obtain its electronic configuration. The electron configuration of a #ca^(2+)# ion. By removing the two outermost electrons from. The calcium ion (ca 2+ ),. Calcium has an atomic number of 20. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
Calcium electron configuration Stock Image C029/5027 Science
By removing the two outermost electrons from. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 1s^2 2s^2 2p^6 3s^2 3p^6. The electron configuration of the calcium ion (ca^2+) is: Simply use this information to obtain its electronic configuration.
Electron Configuration For Calcium
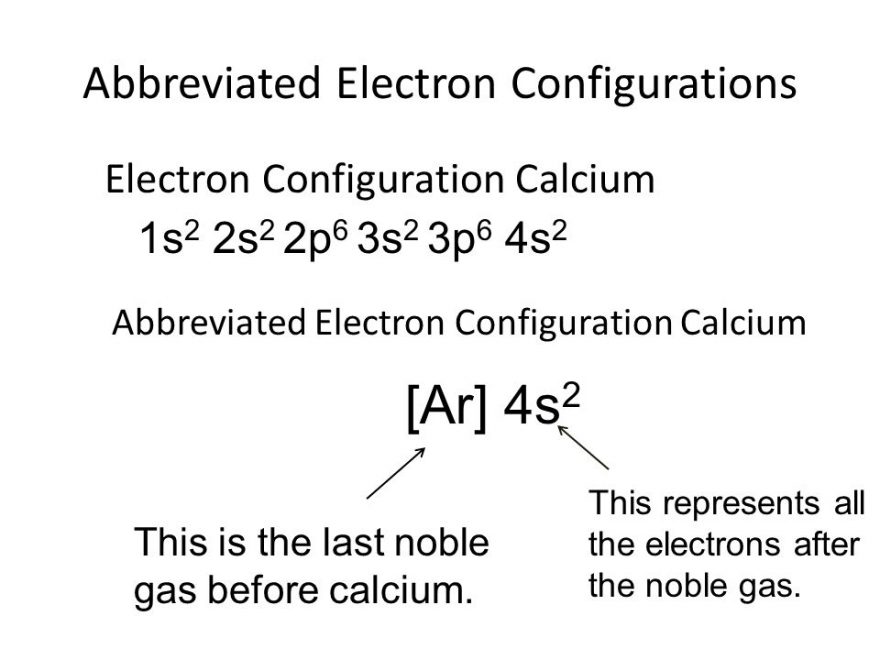
For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The electron configuration of a #ca^(2+)# ion. A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons. The calcium ion (ca 2+ ),. First, we need to know the electron configuration of.
Orbital Diagram For Calcium (Ca) Calcium Electron Configuration
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). The electron configuration of the calcium ion (ca^2+) is: 1s^2 2s^2 2p^6 3s^2 3p^6. Let's determine the electron configuration of a calcium ion,. Simply use this information to obtain its electronic configuration.
Calcium Electron Configuration (Ca) with Orbital Diagram
Let's determine the electron configuration of a calcium ion,. 1s^2 2s^2 2p^6 3s^2 3p^6. By removing the two outermost electrons from. The electron configuration of a #ca^(2+)# ion. The electron configuration of the calcium ion (ca^2+) is:
Electron Configuration For Calcium
The electron configuration of a #ca^(2+)# ion. The calcium ion (ca 2+ ),. Calcium has an atomic number of 20. First, we need to know the electron configuration of a neutral. 1s^2 2s^2 2p^6 3s^2 3p^6.
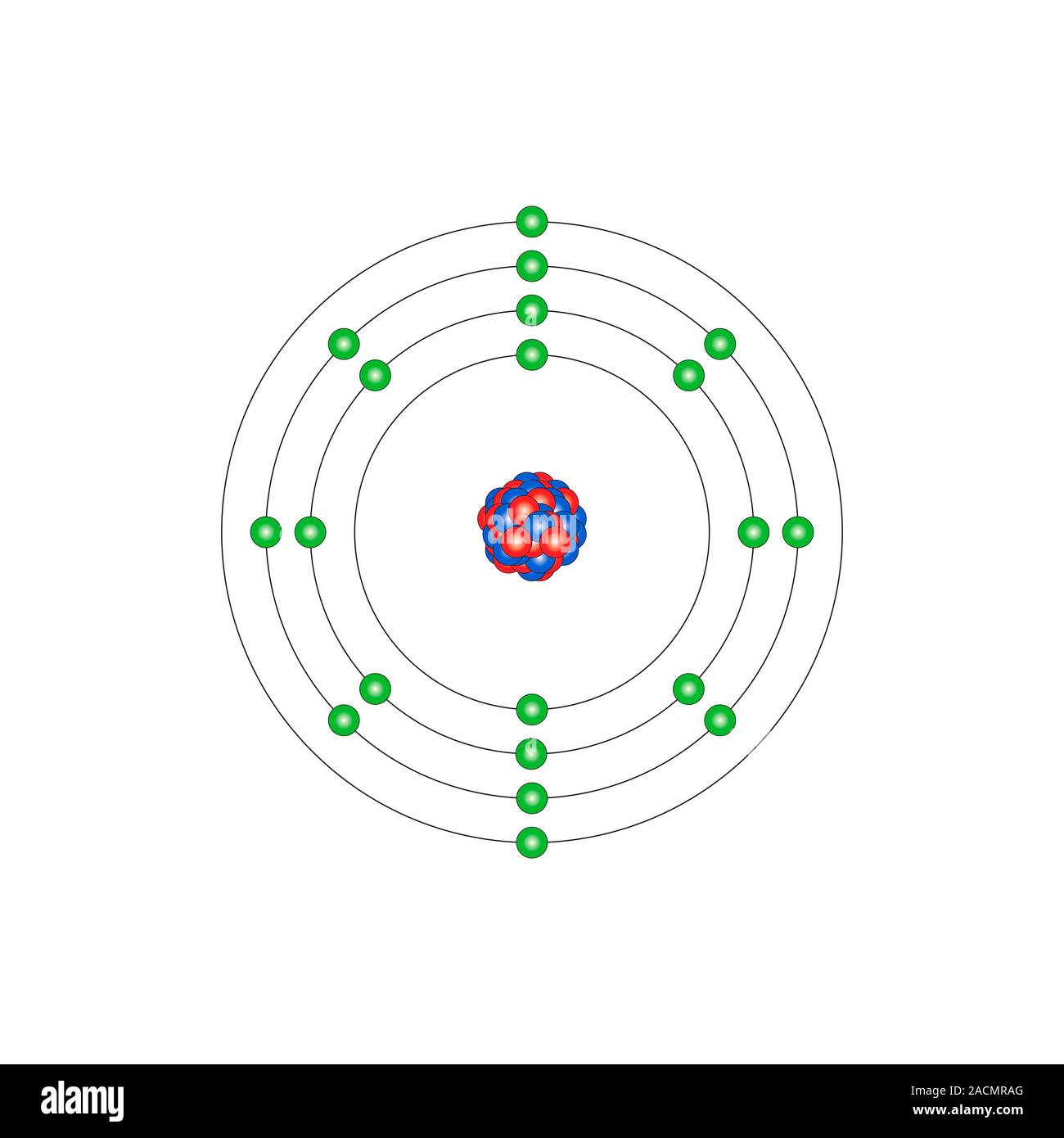
Electron arrangements
The calcium ion (ca 2+ ),. The electron configuration of a #ca^(2+)# ion. Let's determine the electron configuration of a calcium ion,. First, we need to know the electron configuration of a neutral. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
How to Write the Electron Configuration for Calcium (Ca)
First, we need to know the electron configuration of a neutral. By removing the two outermost electrons from. 1s^2 2s^2 2p^6 3s^2 3p^6. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). For instance, the ground state electronic configuration of calcium (z=20) is 1s.
Electron Configuration for Calcium (Ca, Ca2+ ion)
The electron configuration of the calcium ion (ca^2+) is: In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). 1s^2 2s^2 2p^6 3s^2 3p^6. The electron configuration of a #ca^(2+)# ion. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s.
How to Write the Electron Configuration for Calcium (Ca)
Simply use this information to obtain its electronic configuration. First, we need to know the electron configuration of a neutral. Calcium has an atomic number of 20. The electron configuration of the calcium ion (ca^2+) is: A calcium #2+# ion has lost its two valence electrons, and now has #18# electrons.
Electron Configuration For Calcium
In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons). The electron configuration of a #ca^(2+)# ion. The electron configuration of the calcium ion (ca^2+) is: The calcium ion (ca 2+ ),. For instance, the ground state electronic configuration of calcium (z=20) is 1s 2.
A Calcium #2+# Ion Has Lost Its Two Valence Electrons, And Now Has #18# Electrons.
For instance, the ground state electronic configuration of calcium (z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. First, we need to know the electron configuration of a neutral. The electron configuration of the calcium ion (ca^2+) is: By removing the two outermost electrons from.
1S^2 2S^2 2P^6 3S^2 3P^6.
The calcium ion (ca 2+ ),. Let's determine the electron configuration of a calcium ion,. The electron configuration of a #ca^(2+)# ion. In order to write the calcium electron configuration we first need to know the number of electrons for the ca atom (there are 20 electrons).
Simply Use This Information To Obtain Its Electronic Configuration.
Calcium has an atomic number of 20.