What Is The Electron Geometry Of Clf5
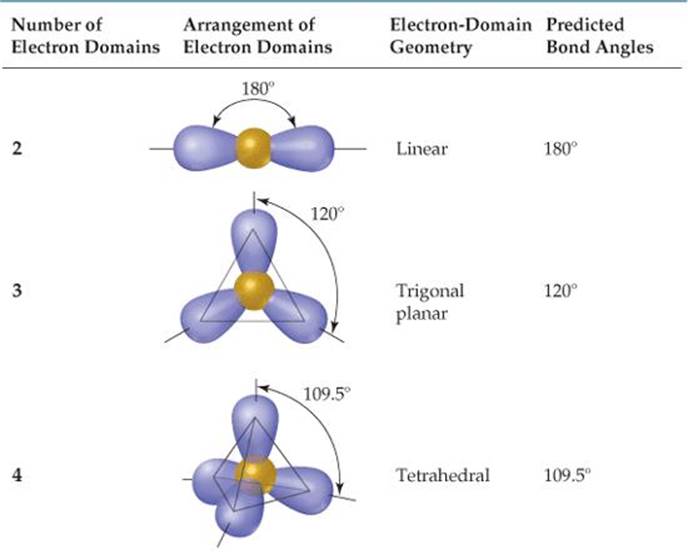
What Is The Electron Geometry Of Clf5 - What is the electronic geometry of clf5? Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. It has a total of seven electron pairs and five atoms. Using the vsepr theory, clf5’s shape is determined by the fact that chlorine has seven electrons in the valence shell and the remaining. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. Electron geometry of clf 5. According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. The electron geometry of the molecule of clf5 is octahedral.
According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. The electron geometry of the molecule of clf5 is octahedral. What is the electronic geometry of clf5? Using the vsepr theory, clf5’s shape is determined by the fact that chlorine has seven electrons in the valence shell and the remaining. Electron geometry of clf 5. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. It has a total of seven electron pairs and five atoms.
Using the vsepr theory, clf5’s shape is determined by the fact that chlorine has seven electrons in the valence shell and the remaining. According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. Electron geometry of clf 5. What is the electronic geometry of clf5? Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. It has a total of seven electron pairs and five atoms. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. The electron geometry of the molecule of clf5 is octahedral.
SOLVEDFrom the electronpair repulsion model, predict the geometry of
The electron geometry of the molecule of clf5 is octahedral. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. Electron geometry of clf 5. It has a total of seven electron pairs and five atoms. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the.
Clf5 Electron Pair Geometry
According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. What is the electronic geometry of clf5? The electron geometry of the molecule of clf5 is octahedral. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic.
Clf5 Electron Pair Geometry
According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. What is the electronic geometry of clf5? It has a total of seven electron pairs and five atoms. Electron geometry of clf 5.
Vsepr Electron Geometry Clf2
Using the vsepr theory, clf5’s shape is determined by the fact that chlorine has seven electrons in the valence shell and the remaining. It has a total of seven electron pairs and five atoms. According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. What is the electronic geometry of clf5? Clf5 has a trigonal bipyramidal.
Solved Draw the Lewis structure for ClF5 in the window below
Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. Electron geometry of clf 5. The electron geometry of the molecule of clf5 is octahedral. According to the vsepr concept, the clf 5 molecule has.
Electron domain geometry bond angle chart booysunshine
Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. It has a total of seven electron pairs and five atoms. According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°..
Molecular geometry
What is the electronic geometry of clf5? The electron geometry of the molecule of clf5 is octahedral. Electron geometry of clf 5. Using the vsepr theory, clf5’s shape is determined by the fact that chlorine has seven electrons in the valence shell and the remaining. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around.
SOLVED Draw the Lewis structure of XeF5+ . What is the overall
What is the electronic geometry of clf5? The electron geometry of the molecule of clf5 is octahedral. According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. Using the vsepr theory, clf5’s shape is determined by the fact.
SOLVEDFrom the electronpair repulsion model, predict the geometry of
Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. Using the vsepr theory, clf5’s shape is determined by the fact that chlorine has seven electrons in the valence shell and the remaining. What is the electronic geometry of clf5? According to the vsepr concept, the clf 5 molecule has an octahedral.
Electron pair geometry chart of asf3 sergdisk
What is the electronic geometry of clf5? The electron geometry of the molecule of clf5 is octahedral. Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. Electron geometry of clf 5. It has a total of seven electron pairs and five atoms.
Using The Vsepr Theory, Clf5’S Shape Is Determined By The Fact That Chlorine Has Seven Electrons In The Valence Shell And The Remaining.
It has a total of seven electron pairs and five atoms. Clf 5 has a square pyramidal molecular geometry and an octahedral electronic shape with bond angles of 90°. Electron geometry of clf 5. According to the vsepr concept, the clf 5 molecule has an octahedral electron geometry.
The Electron Geometry Of The Molecule Of Clf5 Is Octahedral.
Clf5 has a trigonal bipyramidal geometry, where the five fluorine atoms are symmetrically arranged around the chlorine. What is the electronic geometry of clf5?