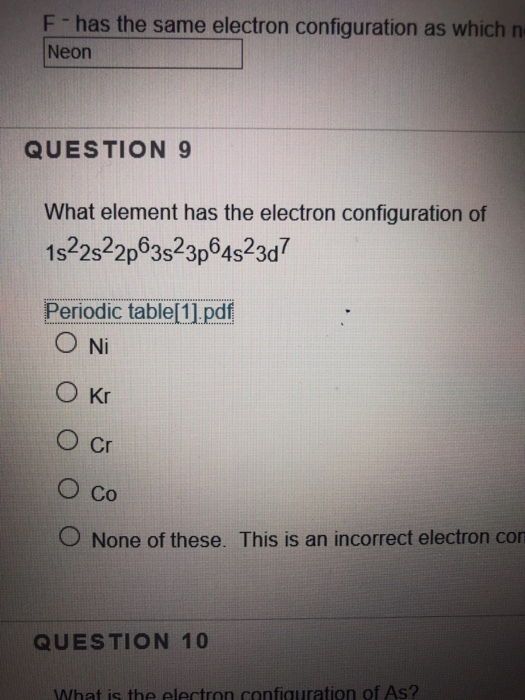
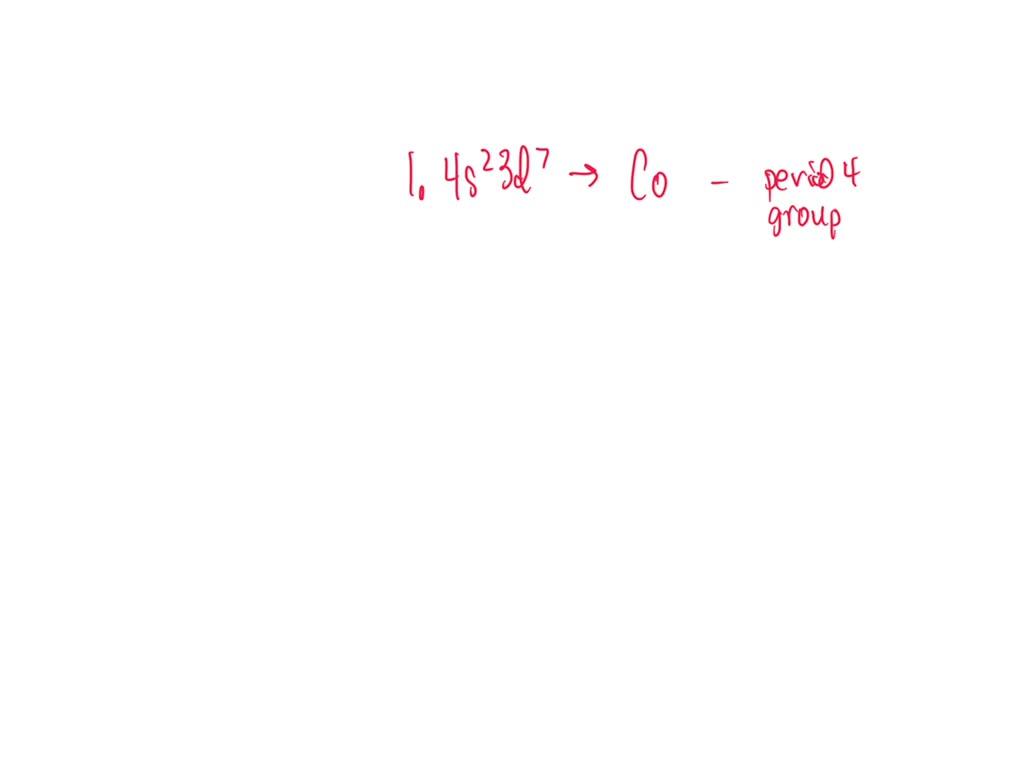What Is The Element With An Electron Configuration Of 1S22S22P63S23P64S23D7
What Is The Element With An Electron Configuration Of 1S22S22P63S23P64S23D7 - What element has the electron configuration 1s22s22p23s23p2? What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? Remember that for neutral atoms,. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. The element with this electron configuration is carbon (c). The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. The number of protons must be the same as the element is electrically neutral. Just add up the electrons. Then number of protons =. If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration.
What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. What element has the electron configuration 1s22s22p23s23p2? The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. The element with this electron configuration is carbon (c). The number of protons must be the same as the element is electrically neutral. Remember that for neutral atoms,. Just add up the electrons. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. There are 2 steps to solve this one.
There are 2 steps to solve this one. What element has the electron configuration 1s22s22p23s23p2? Then number of protons =. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? Remember that for neutral atoms,. The number of protons must be the same as the element is electrically neutral. The element with this electron configuration is carbon (c). The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Just add up the electrons. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d.
Electron Configuration Of Arsenic
The element with this electron configuration is carbon (c). Remember that for neutral atoms,. Then number of protons =. The number of protons must be the same as the element is electrically neutral. If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration.
(1) The element with an electron configuration of 1s22s22p63s23p64s23d7
Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. There are 2 steps to solve this one. The element with this electron configuration is carbon (c). The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is.
Electronic Configuration Antimony Learn Important Terms and Concepts
What element has the electron configuration 1s22s22p23s23p2? The number of protons must be the same as the element is electrically neutral. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element.
[Solved] Which element has the following electron configuration
There are 2 steps to solve this one. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Just add up the electrons. The element with this electron configuration is carbon (c). Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2.
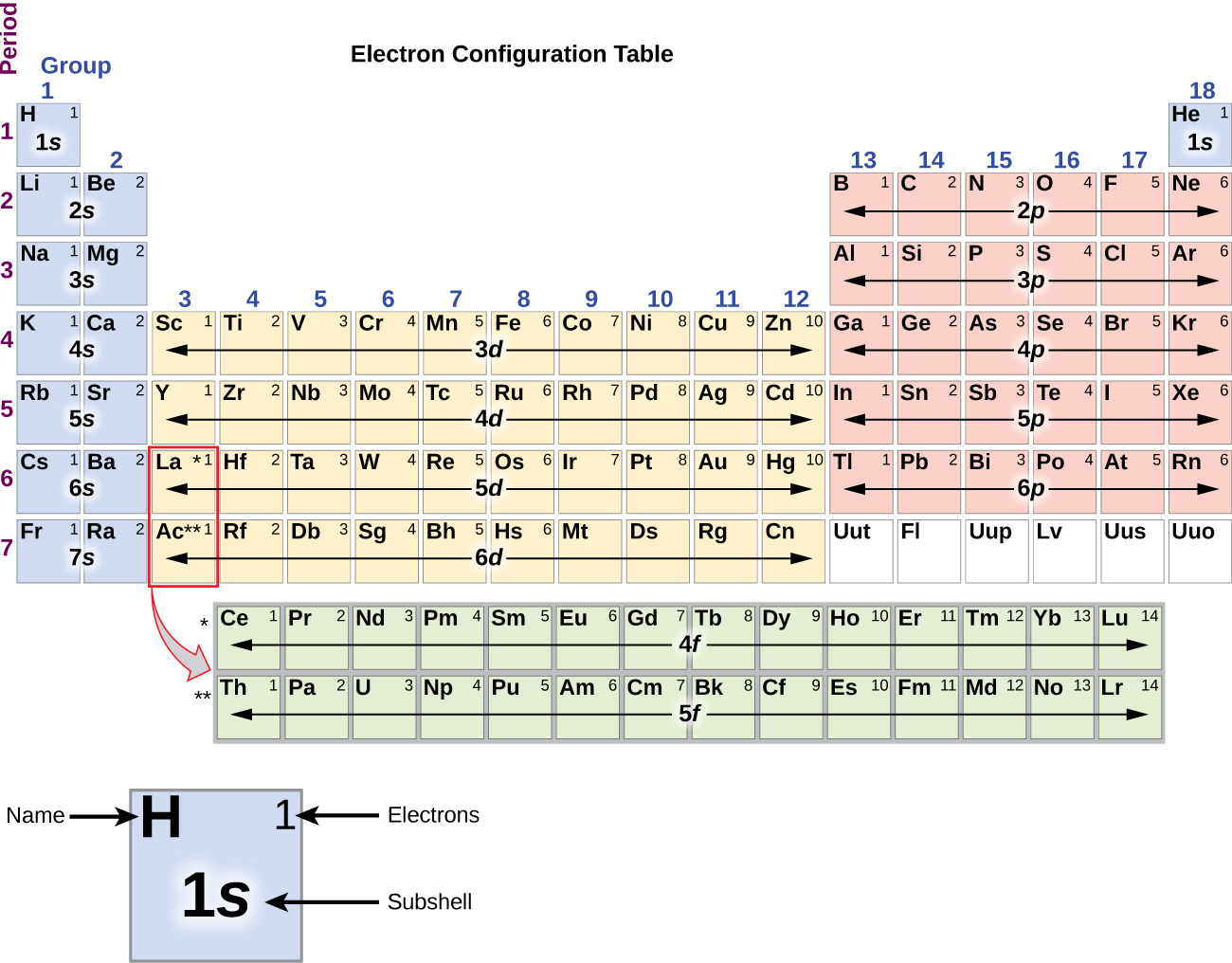
Electron Configuration of Elements Chemistry Periodic Table
The element with this electron configuration is carbon (c). Just add up the electrons. Remember that for neutral atoms,. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. There are 2 steps to solve this one.
1.5 Electronic Structure of Atoms (Electron Configurations)
Just add up the electrons. The number of protons must be the same as the element is electrically neutral. What element has the electron configuration 1s22s22p23s23p2? The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. Remember that for neutral atoms,.
5.2 Electronic Structure of Atoms (Electron Configurations
Just add up the electrons. What element has the electron configuration 1s22s22p23s23p2? If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. The.
use the periodic table to identify the element indicated by each
Identify the total number of electrons in the given electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d. Just add up the electrons. Remember that for neutral atoms,. There are 2 steps to solve this one. The element with this electron configuration is carbon (c).
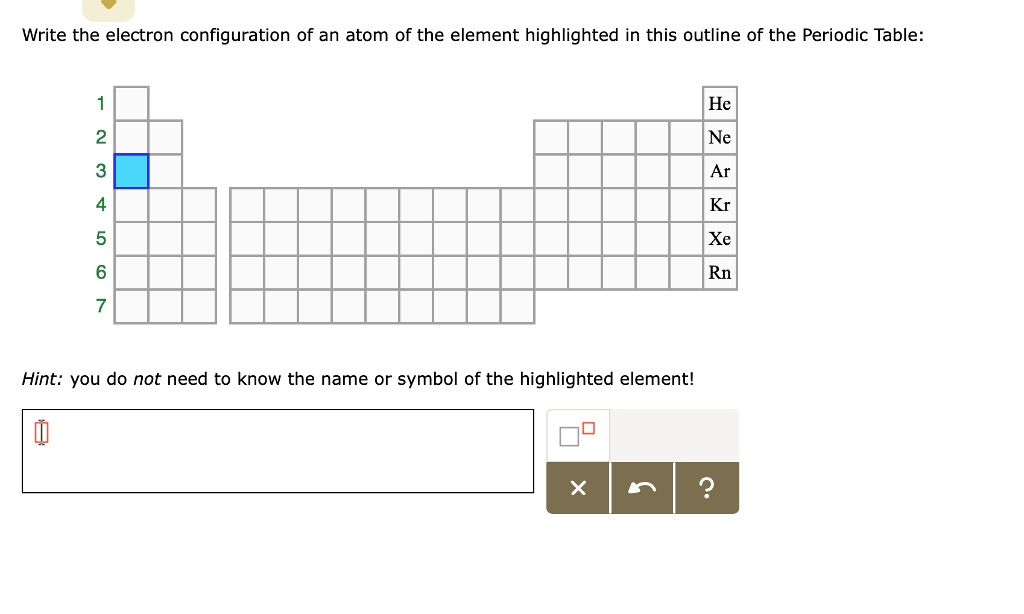
write the electron configuration of an atom of the element highlighted
If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. Remember that for neutral atoms,. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. The number of protons must be the same as the element is electrically neutral. Just add up the electrons.
Solved F has the same electron configuration as which n
Then number of protons =. The electron configuration 1s22s22p63s23p64s23d7 corresponds to the element manganese (mn), which is in group 7. What element has the ground state electron configuration 1s22s22p63s23p64s23d7 ? The element with this electron configuration is carbon (c). What element has the electron configuration 1s22s22p23s23p2?
The Electron Configuration 1S22S22P63S23P64S23D7 Corresponds To The Element Manganese (Mn), Which Is In Group 7.
What element has the electron configuration 1s22s22p23s23p2? If you are referring to a neutral atom, then vanadium (v) has that particular electron configuration. Just add up the electrons. The element with this electron configuration is carbon (c).
Identify The Total Number Of Electrons In The Given Electron Configuration 1 S 2 2 S 2 2 P 6 3 S 2 3 P 6 4 S 2 3 D.
There are 2 steps to solve this one. Remember that for neutral atoms,. The number of protons must be the same as the element is electrically neutral. Then number of protons =.