What Is The Hybridization Of H2O
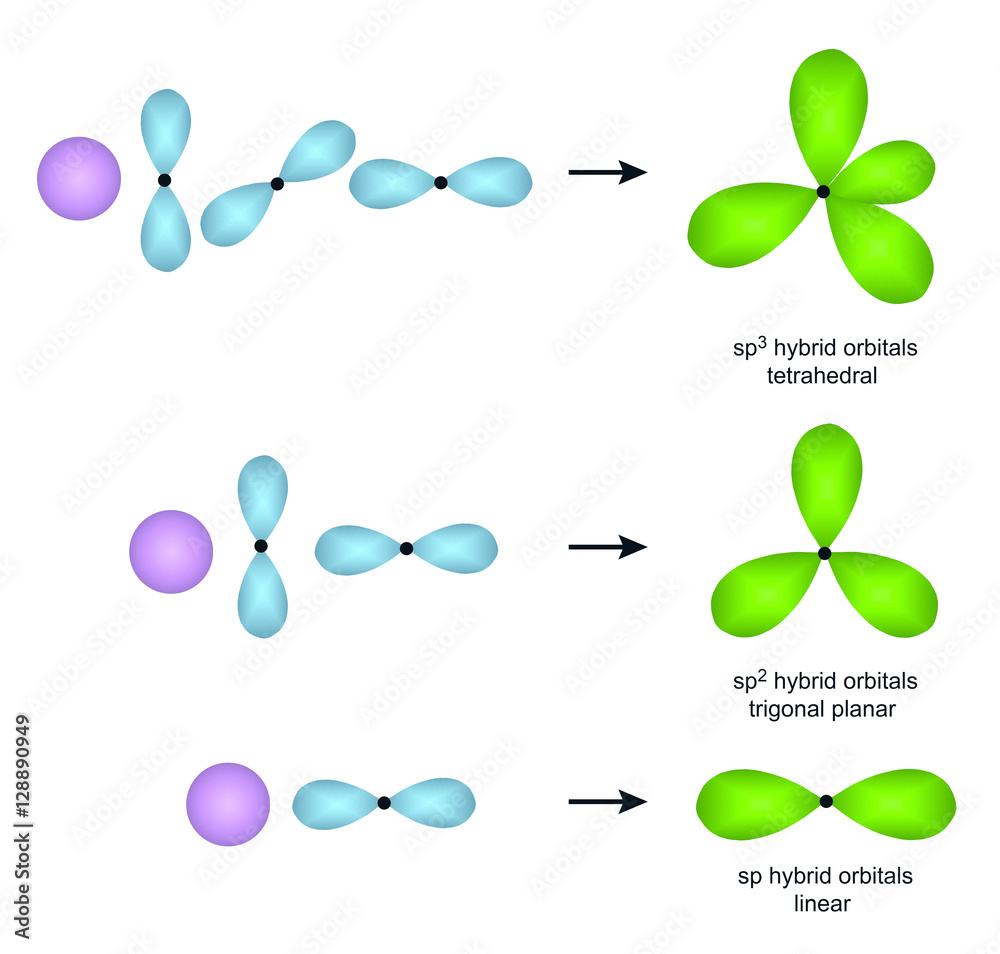
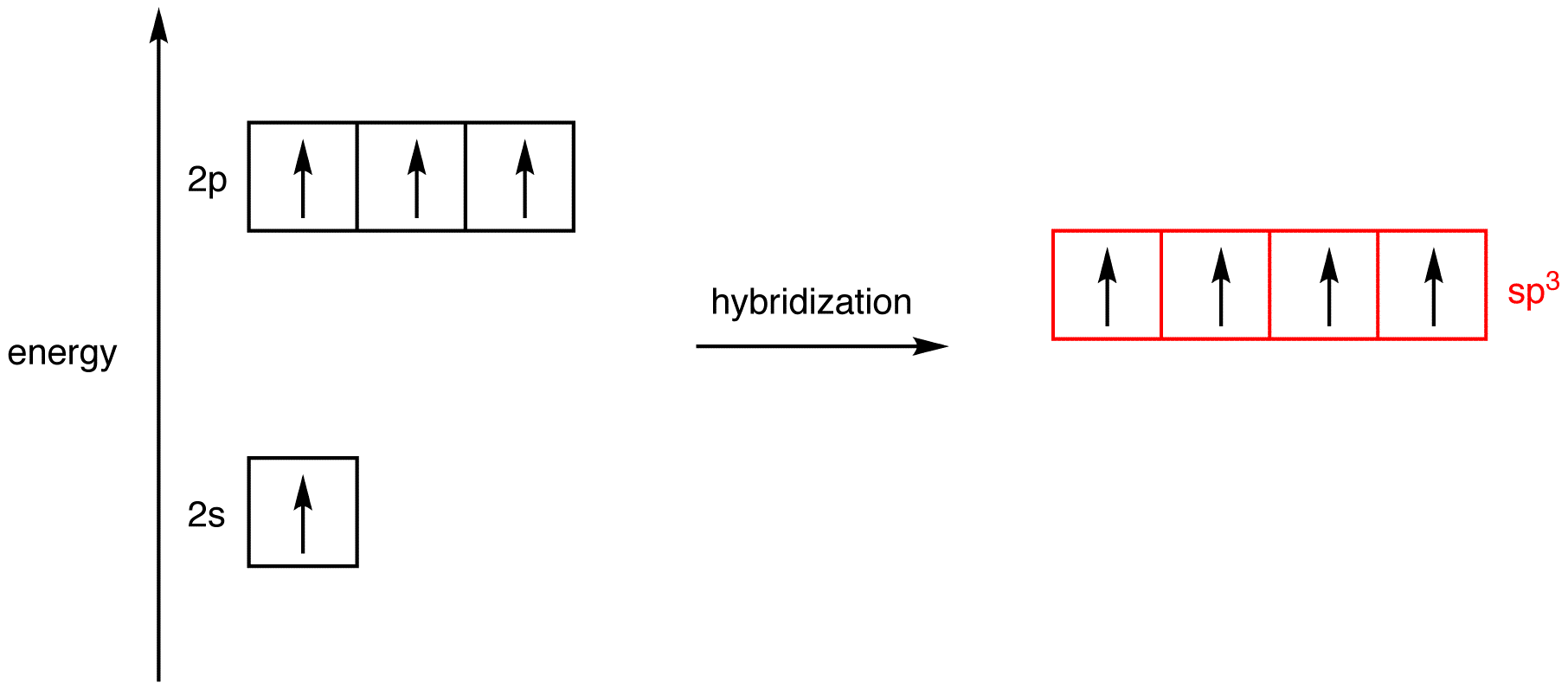
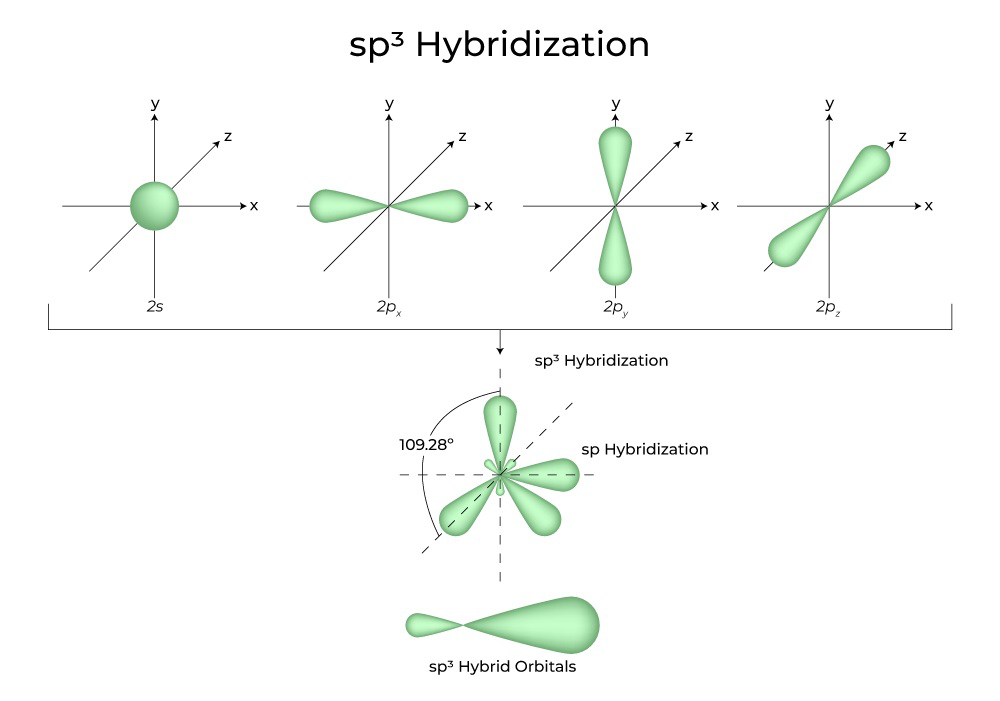
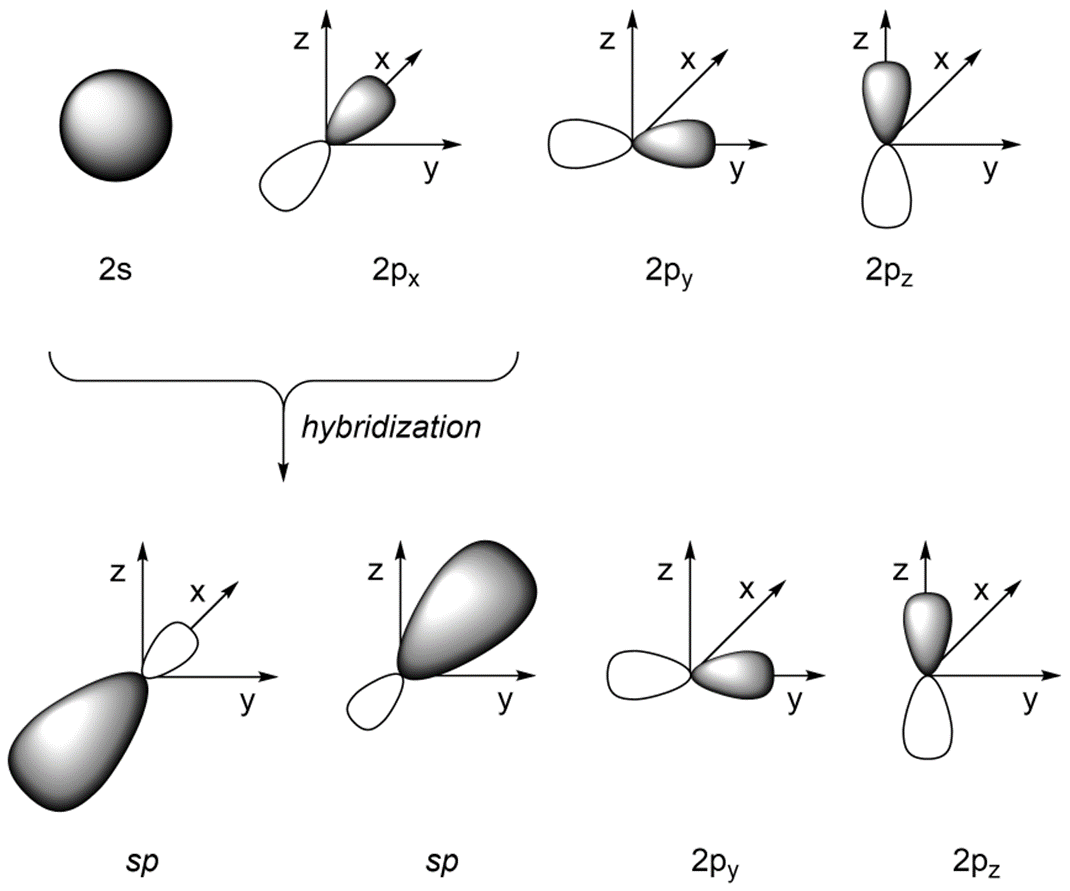
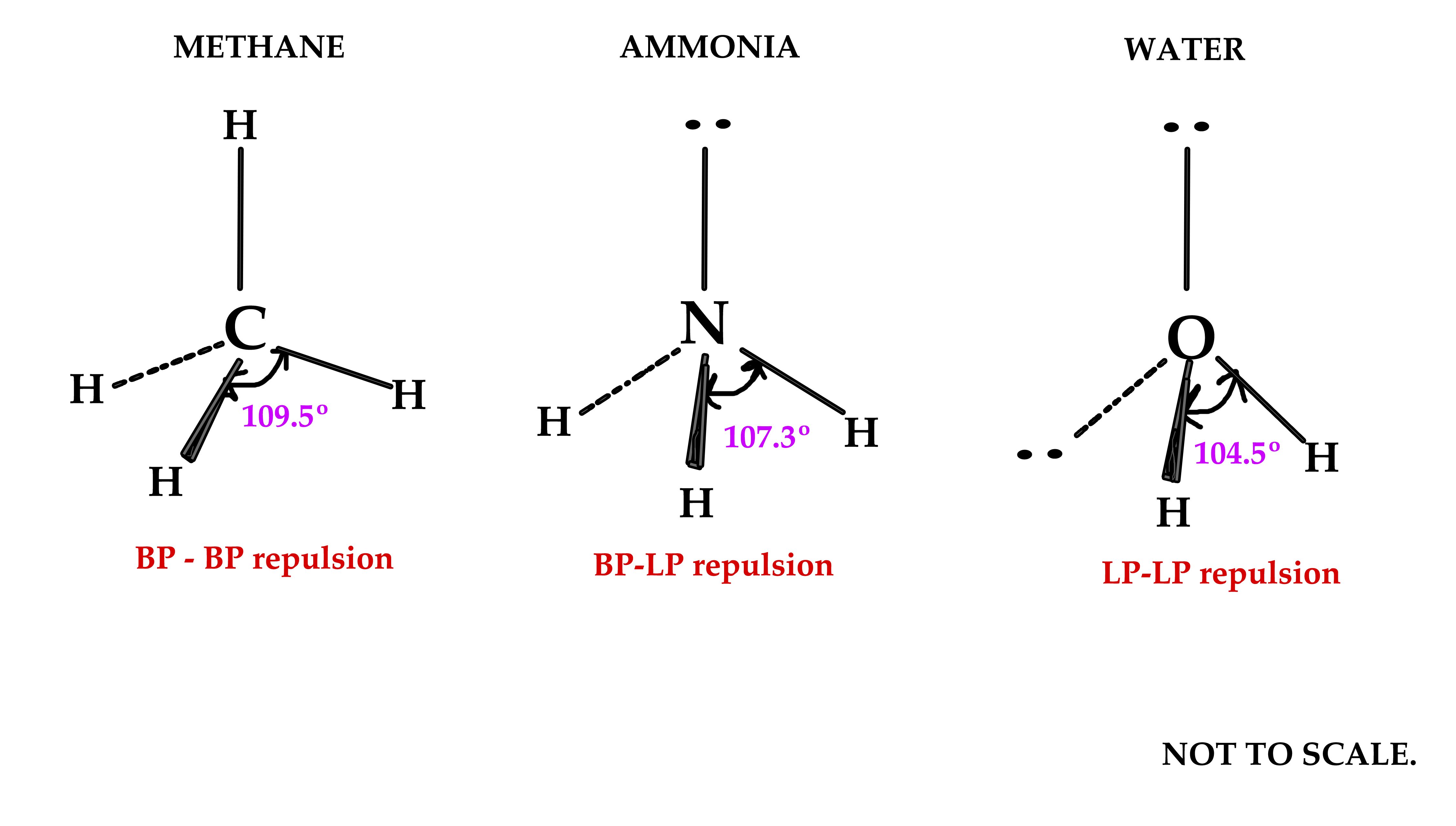
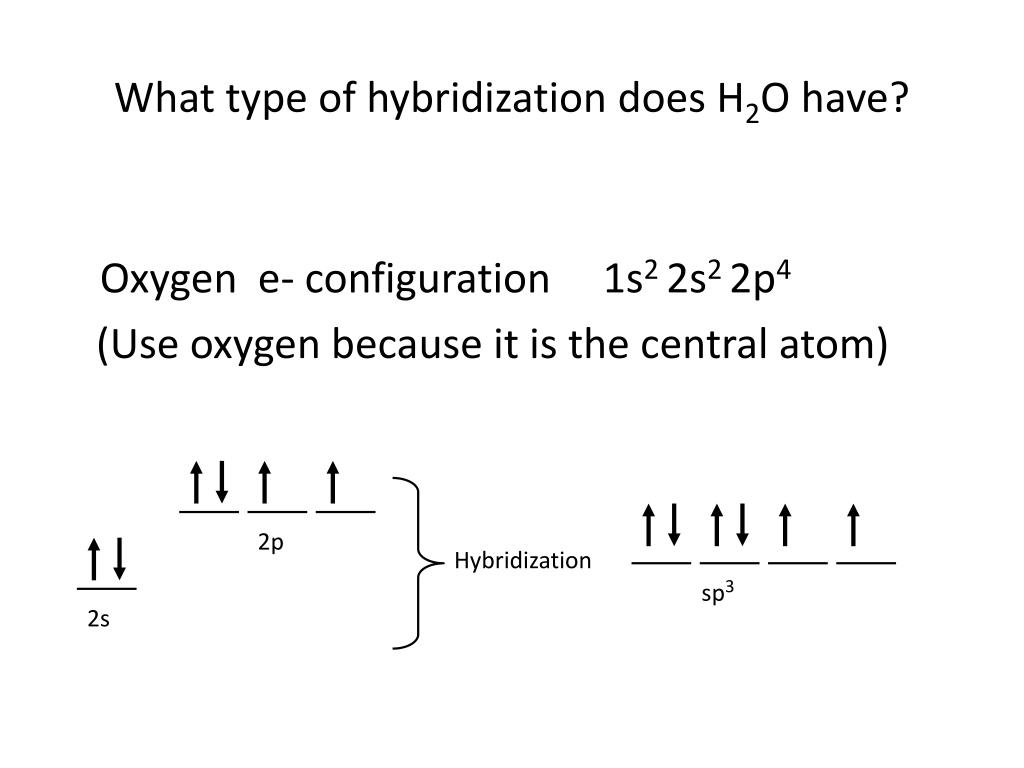
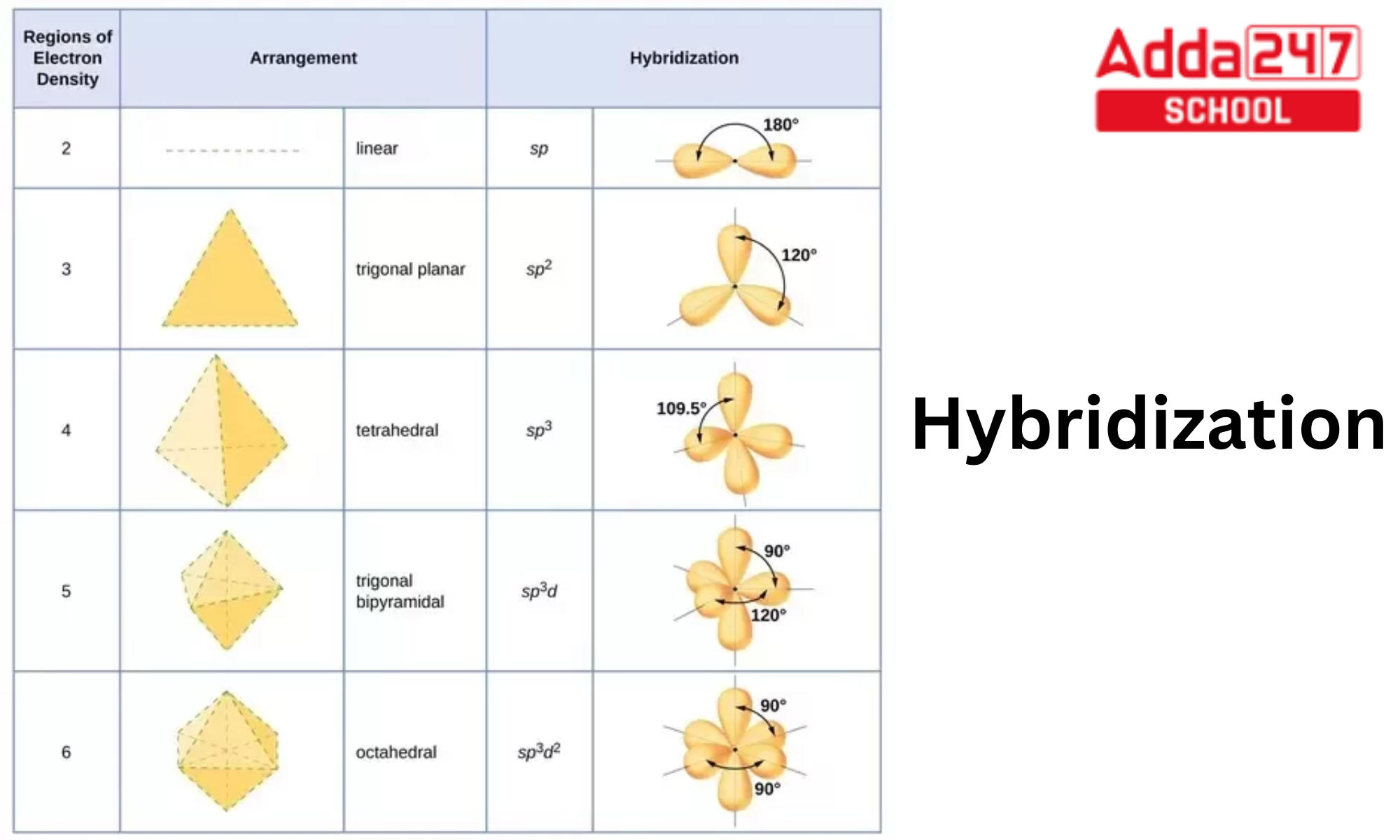
What Is The Hybridization Of H2O - This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. The hybridization of water (h2o) is sp3.
This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and.
The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. The hybridization of water (h2o) is sp3. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three.
Hybridization Definition, Types, Rules, Examples, 41 OFF
The hybridization of water (h2o) is sp3. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and.
Hybridization Types and Examples of Hybridization
This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and.
Hybridization Definition, Types, Rules, Examples, 58 OFF
The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. The hybridization of water (h2o) is sp3. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three.
Hybridization Definition, Types, Rules, Examples, 58 OFF
This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and.
Hybridization reduces vulnerability to climate change
The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3.
Hybridization Chemistry LibreTexts
The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. The hybridization of water (h2o) is sp3. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three.
Hybridization Chemistry
This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. The hybridization of water (h2o) is sp3.
PPT Hybridization PowerPoint Presentation, free download ID2109983
The hybridization of water (h2o) is sp3. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and.
What is Hybridization? sp3, sp2, Examples and Formula
The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3.
Hybridization Definition, Types, Rules, Examples
The hybridization of water (h2o) is sp3, as it has four regions of electron density around the oxygen atom (two bonding pairs and. This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3.
The Hybridization Of Water (H2O) Is Sp3, As It Has Four Regions Of Electron Density Around The Oxygen Atom (Two Bonding Pairs And.
This means that the oxygen atom in water forms four hybrid orbitals by combining one 2s and three. The hybridization of water (h2o) is sp3.