What Is The Hybridization Of Nitrogen In No2
What Is The Hybridization Of Nitrogen In No2 - As per the hybridization rule, the sum of the number of. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. Two of the sp 3 hybridized orbitals overlap with s orbitals from. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The simple way to determine the hybridization of no 2 is by. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals.
In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. Two of the sp 3 hybridized orbitals overlap with s orbitals from. As per the hybridization rule, the sum of the number of. The simple way to determine the hybridization of no 2 is by. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals.
In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. As per the hybridization rule, the sum of the number of. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. The simple way to determine the hybridization of no 2 is by. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals.
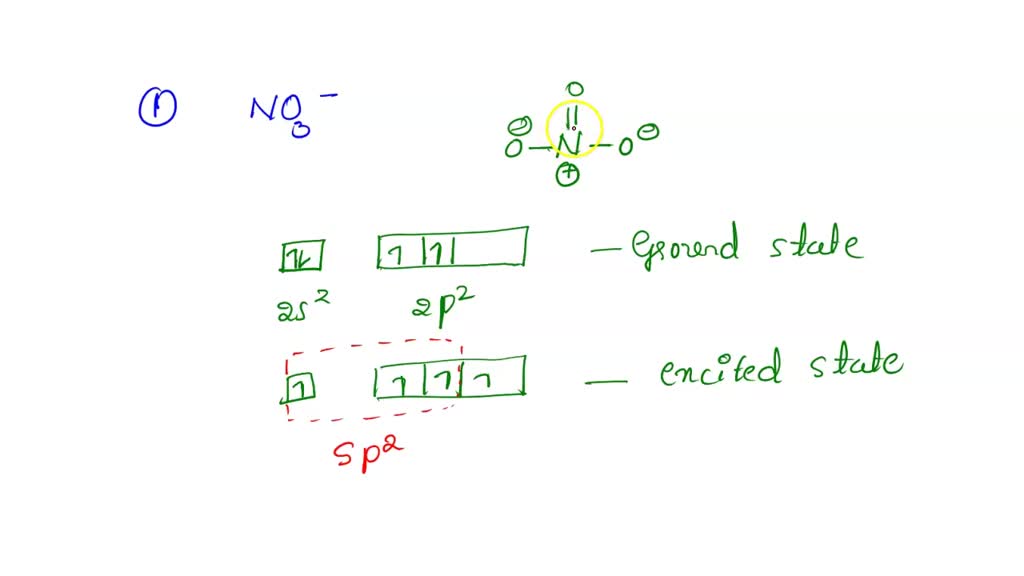
3. The hybridization of atomic orbitals of nitrogen in NO2+ ,NO2− and NH4..
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The simple way to determine the hybridization of no 2 is by. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The hybridization of nitrogen varies from.
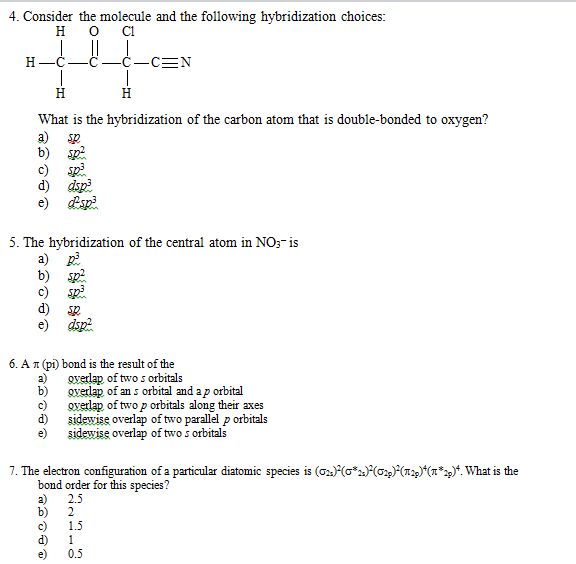
[Solved] Identify how many N (NITROGEN) atoms have sp2
Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. As per the hybridization.
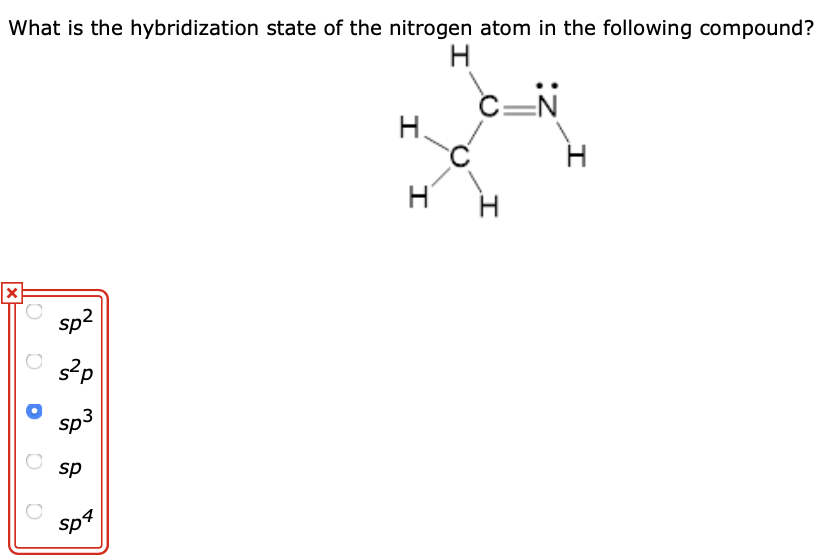
Solved What is the hybridization state of the nitrogen atom
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The simple way to determine the hybridization of no 2 is by. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. As per the hybridization rule, the sum of the number of. The hybridization of nitrogen varies.
Solved 1. The hybridization of the nitrogen atom inthe
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. As per the hybridization rule, the sum of the number of. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. Two of.
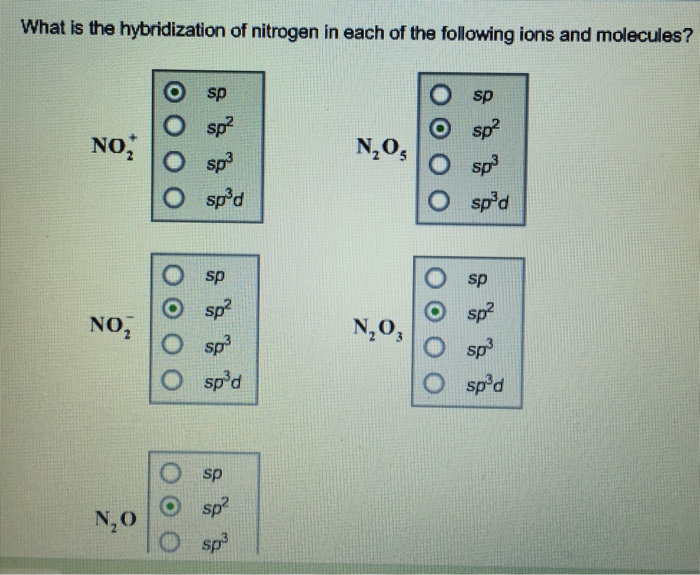
What s the hybridization of nitrogen in each of the
The simple way to determine the hybridization of no 2 is by. As per the hybridization rule, the sum of the number of. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals..
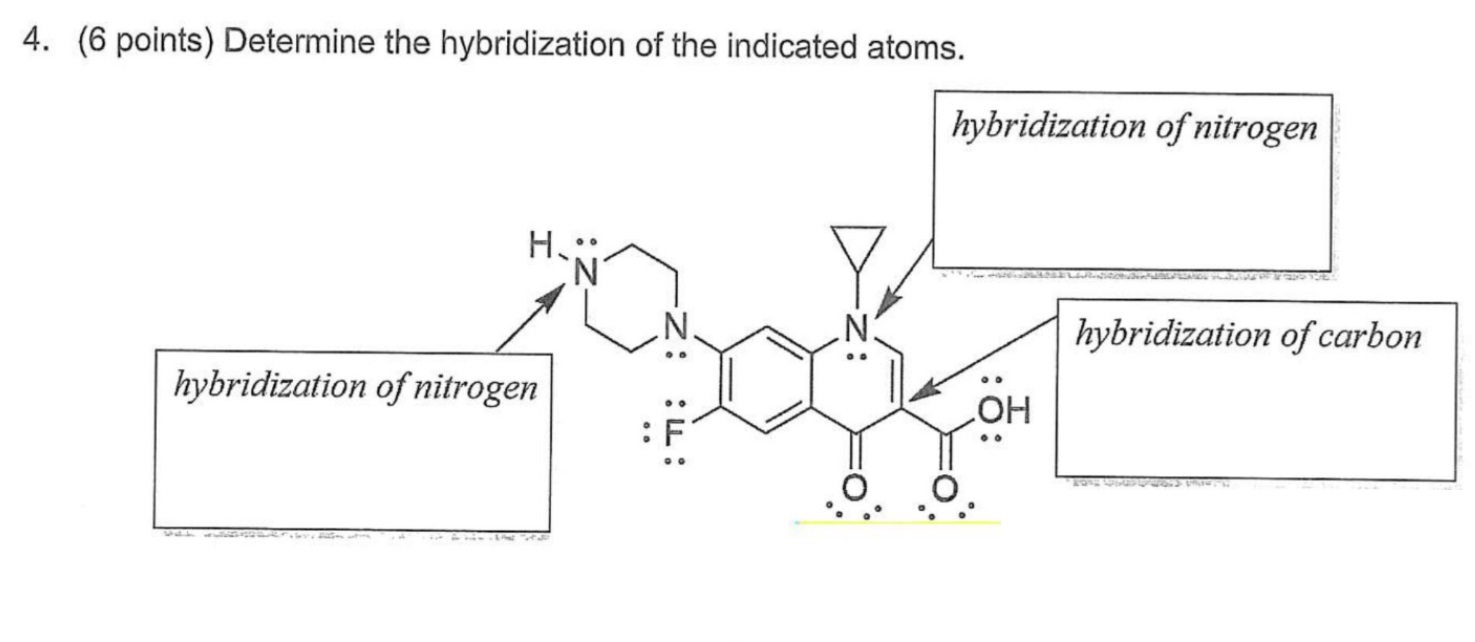
Solved 4. (6 points) Determine the hybridization of the
The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. The simple way to determine the hybridization of no 2 is by. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. As per the hybridization rule, the sum of the number of. In no 2, around the nitrogen.
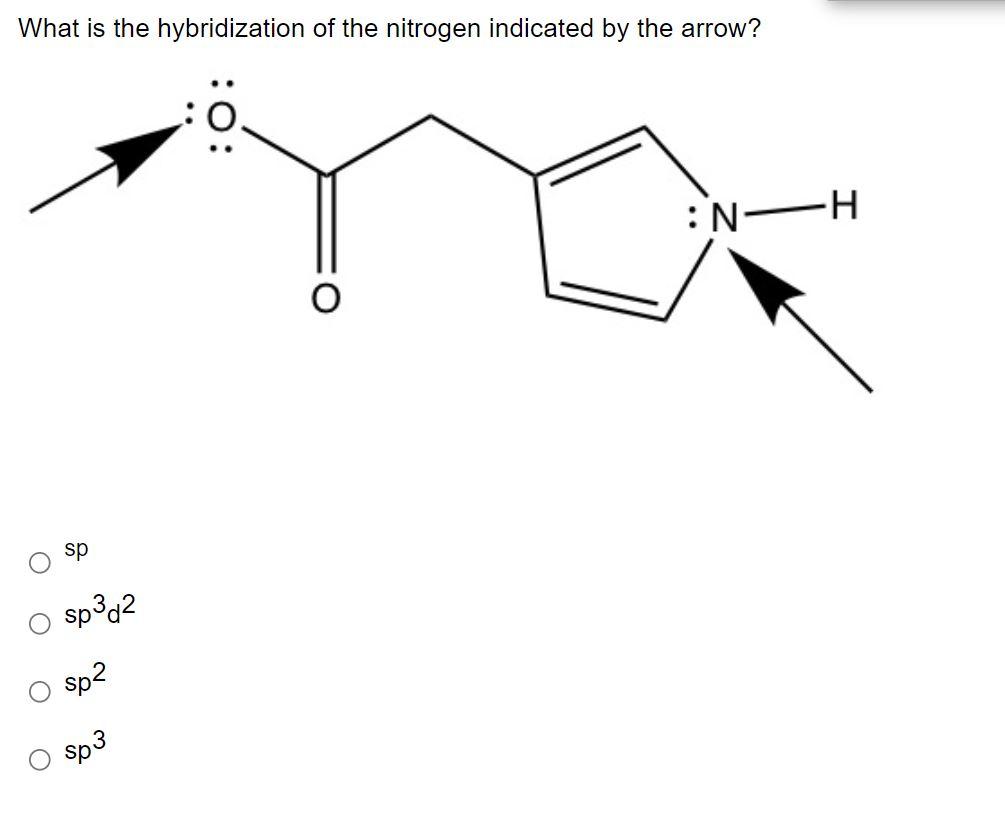
Solved What is the hybridization of the nitrogen indicated
The simple way to determine the hybridization of no 2 is by. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. Two of the sp 3 hybridized orbitals overlap with s orbitals from. As per the hybridization rule, the sum of the number of. Nitrogen dioxide (no 2).
The hybridization of atomic orbital of nitrogen in NO2+, NO3 and NH4
As per the hybridization rule, the sum of the number of. Two of the sp 3 hybridized orbitals overlap with s orbitals from. Nitrogen dioxide (no 2) involves an sp 2 hybridization type. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The hybridization of nitrogen varies from sp to sp3 depending on.
SOLVED What is the hybridization of nitrogen in the nitrite ion
The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. In no 2, around the nitrogen atom, there are two sigma bonds and one unshared electron. The simple way to determine the hybridization.
Solved What is the hybridization of the nitrogen atom in
Nitrogen dioxide (no 2) involves an sp 2 hybridization type. Two of the sp 3 hybridized orbitals overlap with s orbitals from. The simple way to determine the hybridization of no 2 is by. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. In no 2, around the nitrogen atom, there are two.
Two Of The Sp 3 Hybridized Orbitals Overlap With S Orbitals From.
The hybridization of nitrogen varies from sp to sp3 depending on the molecular structure and the number of bonded regions and. As per the hybridization rule, the sum of the number of. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The simple way to determine the hybridization of no 2 is by.
In No 2, Around The Nitrogen Atom, There Are Two Sigma Bonds And One Unshared Electron.
Nitrogen dioxide (no 2) involves an sp 2 hybridization type.
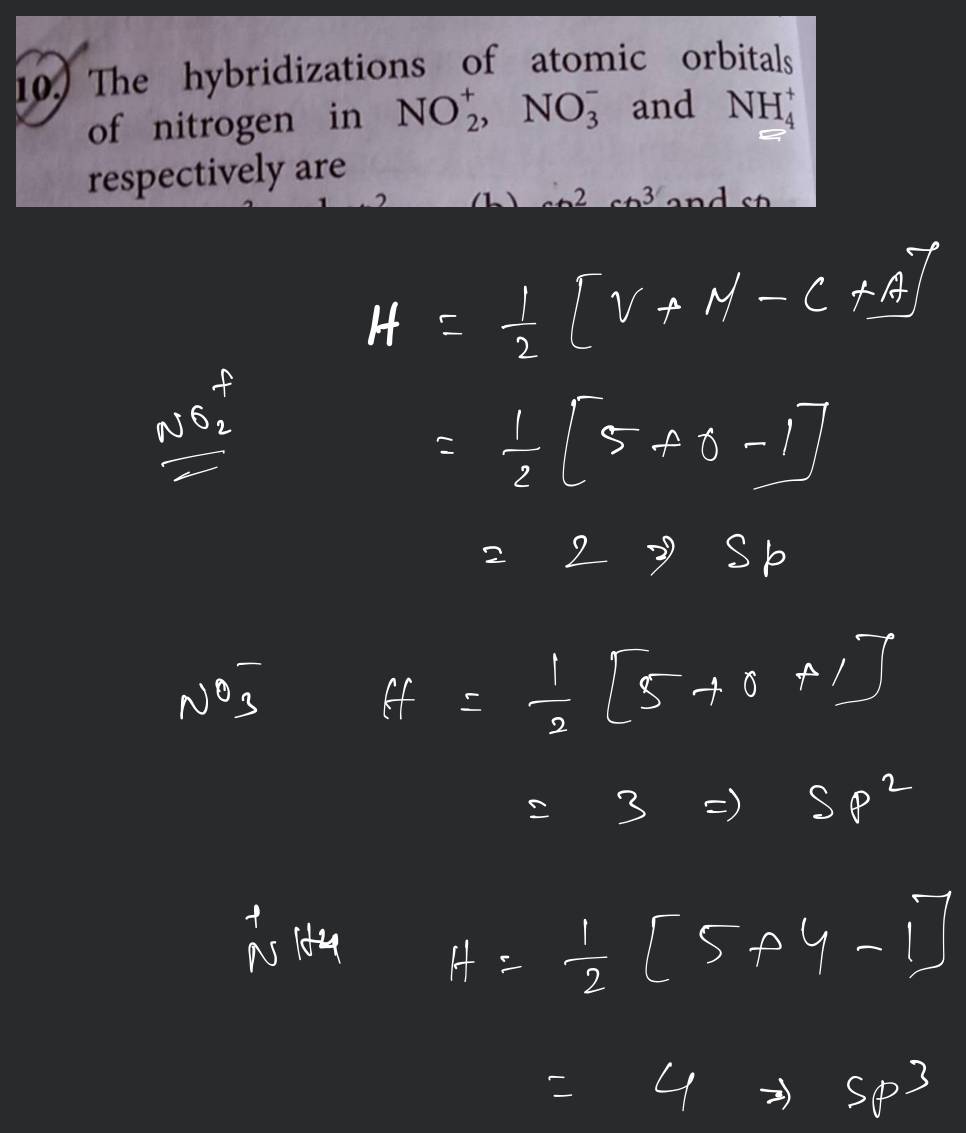
![[Solved] Identify how many N (NITROGEN) atoms have sp2](https://media.cheggcdn.com/media/250/25001471-53e2-40e1-97ef-668977575fc1/phppbkvdZ)