What Is The Hybridization Of The Central Atom In Sf6
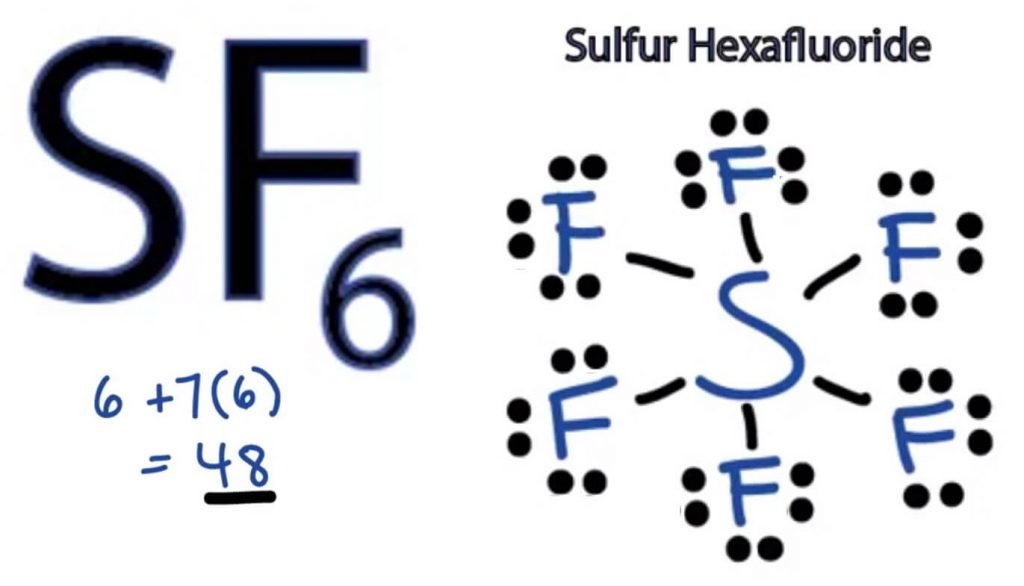
What Is The Hybridization Of The Central Atom In Sf6 - Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. A molecule of sulfur hexafluoride. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization.
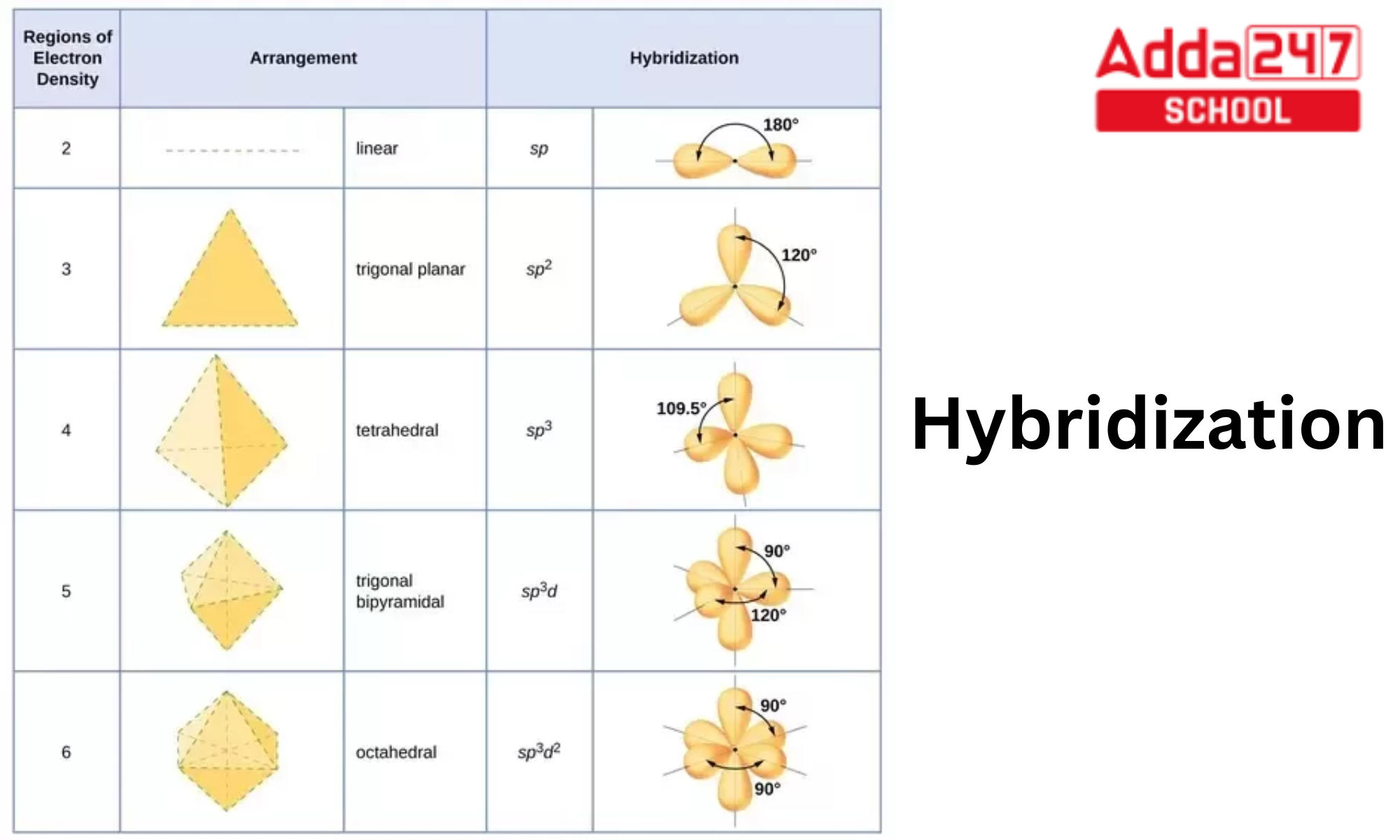
The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. A molecule of sulfur hexafluoride. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during.
The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. A molecule of sulfur hexafluoride. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization.
La geometría molecular SF6, la Estructura, la Forma y la polaridad de
The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2.
sp, sp2, sp3 Hybridization Examples, sp3d2 Shape & Structure
In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. Now that we know the lewis structure of sf6, we.
Hybridization of SF6 Molecule
A molecule of sulfur hexafluoride. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from.
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization
Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. A molecule of sulfur hexafluoride. The central.
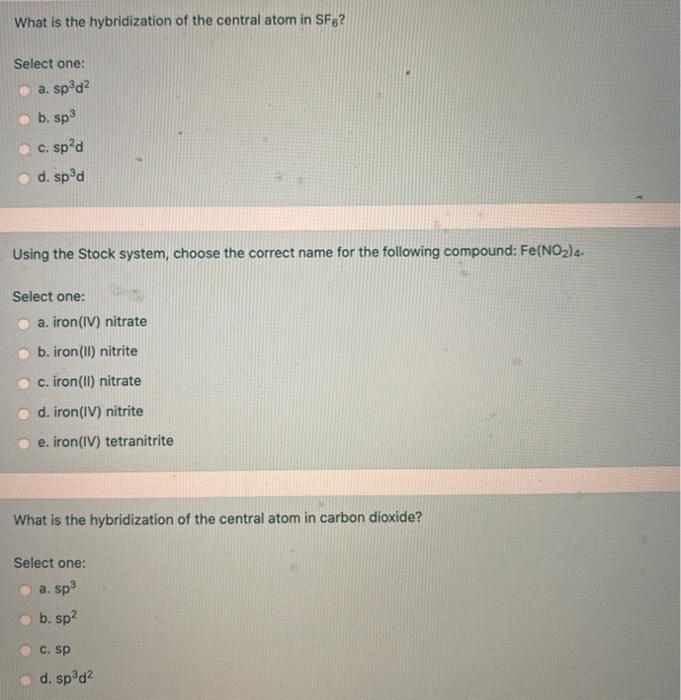
Solved What is the hybridization of the central atom in SF6?
The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. In s.
Solved sf6 lewis structure hybridization of central atom
The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds created during. A molecule of sulfur hexafluoride. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex],.
Hybridization and Hybrid Orbitals ChemTalk
The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. A molecule of sulfur hexafluoride. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. In s f 6 the central sulphur atom.
What is the hybridization of the central atom in \ce{SF6}? Quizlet
Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The orbitals involved in sulphur hexafluoride hybridization, as well as.
SF6 Lewis结构,分子几何,杂交,和MO图技术科学家万博网页版 万博网页版,万博体育app手机版登录
In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. A molecule of sulfur hexafluoride. The central s atom in the sf 6 molecule is sp 3 d 2 hybridized. The electronic configuration of sulfur is.
Ceo 2024 Sf6 Hybridization Kally Mahala
Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The orbitals involved in sulphur hexafluoride hybridization, as well as the bonds.
The Orbitals Involved In Sulphur Hexafluoride Hybridization, As Well As The Bonds Created During.
In s f 6 the central sulphur atom has the ground state configuration, 3 s 2 3 p 4 one electron each from 3 s and 3 p orbitals is promoted to 3 d. The electronic configuration of sulfur is 1s 2 2s 2 2p 6 3s 2 3p 4. The sulfur atom in sulfur hexafluoride, [latex]\ce{sf6}[/latex], exhibits sp 3 d 2 hybridization. Now that we know the lewis structure of sf6, we can now determine the atoms’ hybridization in the molecule.
The Central S Atom In The Sf 6 Molecule Is Sp 3 D 2 Hybridized.
A molecule of sulfur hexafluoride.