What Is The Hybridization Of The Central Atom In So3
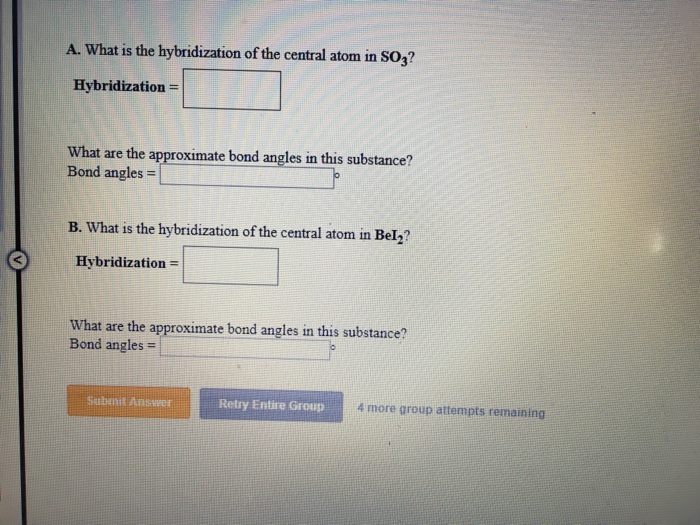
What Is The Hybridization Of The Central Atom In So3 - In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. Between each atom, we have double bonds. One is a sigma bond (σ), and the other one is the pi. As you can see in the structure; This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. The hybridization of the central atom in so3 is sp2.
In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. Between each atom, we have double bonds. This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. As you can see in the structure; One is a sigma bond (σ), and the other one is the pi. The hybridization of the central atom in so3 is sp2.
One is a sigma bond (σ), and the other one is the pi. Between each atom, we have double bonds. The hybridization of the central atom in so3 is sp2. As you can see in the structure; This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to.
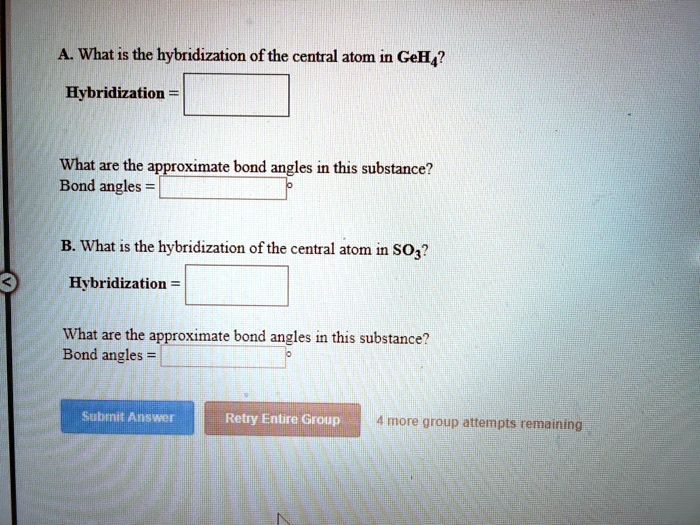
SOLVED What is the hybridization of the central atom in GeH
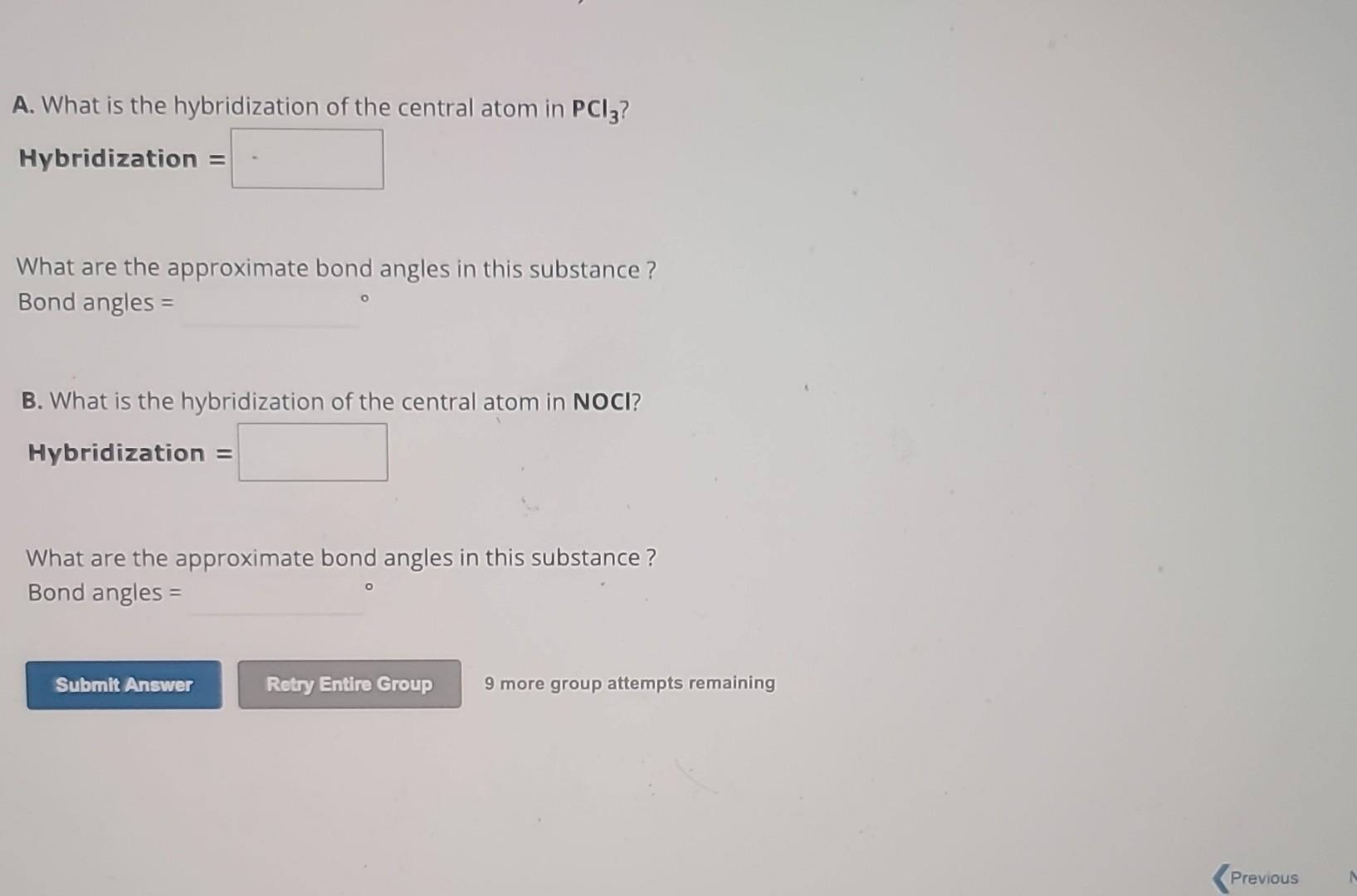
In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. The hybridization of the central atom in so3 is sp2. As you can see in the structure; Between each atom, we have double bonds. This means that the sulfur atom has three hybrid orbitals that are involved in bonding.
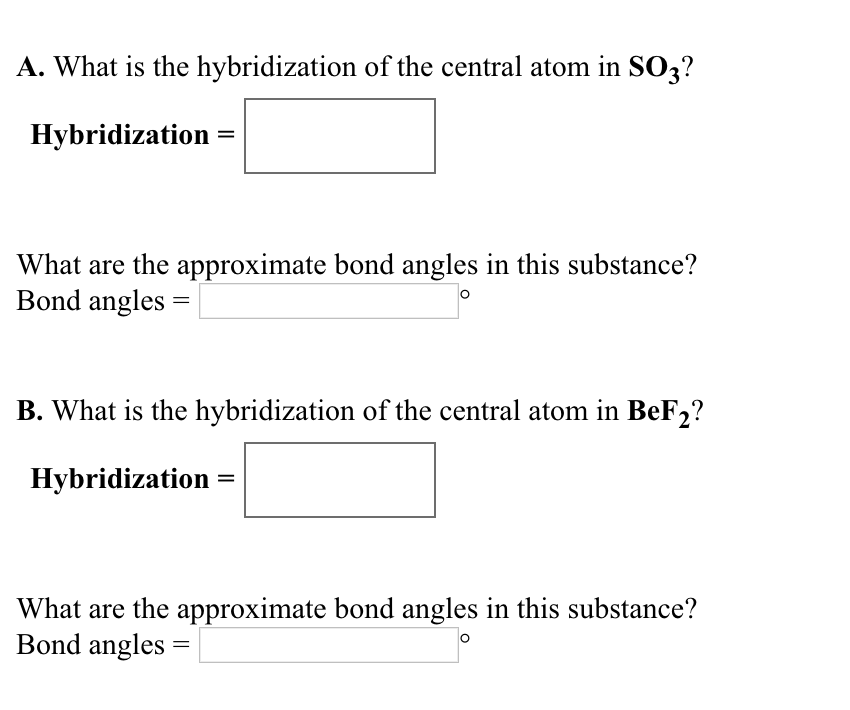
Solved A. What is the hybridization of the central atom in
In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. One is a sigma bond (σ), and the other one is the pi. The hybridization of the central atom in so3 is sp2. As you can see in the structure; Between each atom, we have double bonds.
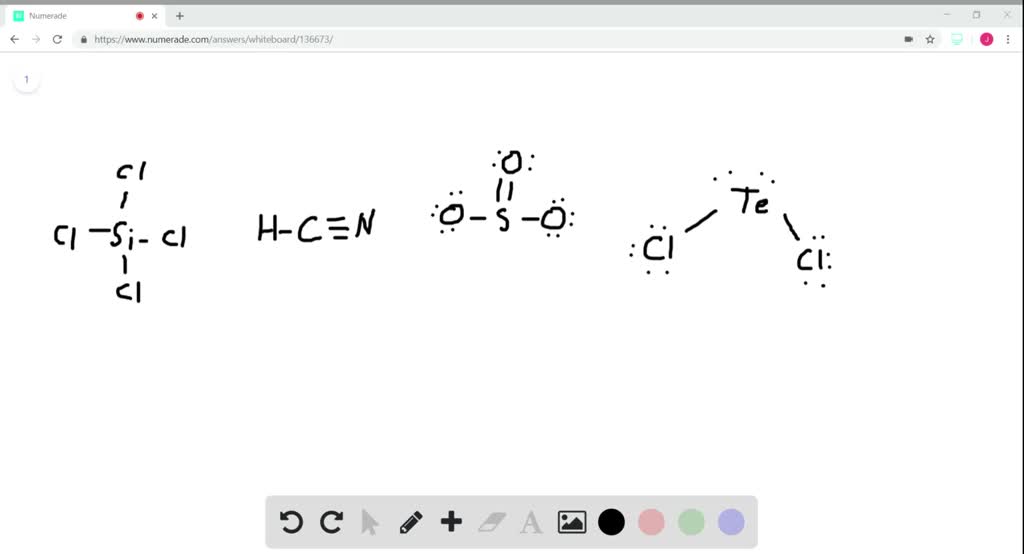
SOLVEDWhat is the hybridization of the central atom in (a) SiCl4, (𝐛
The hybridization of the central atom in so3 is sp2. One is a sigma bond (σ), and the other one is the pi. Between each atom, we have double bonds. This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. As you can see in the structure;
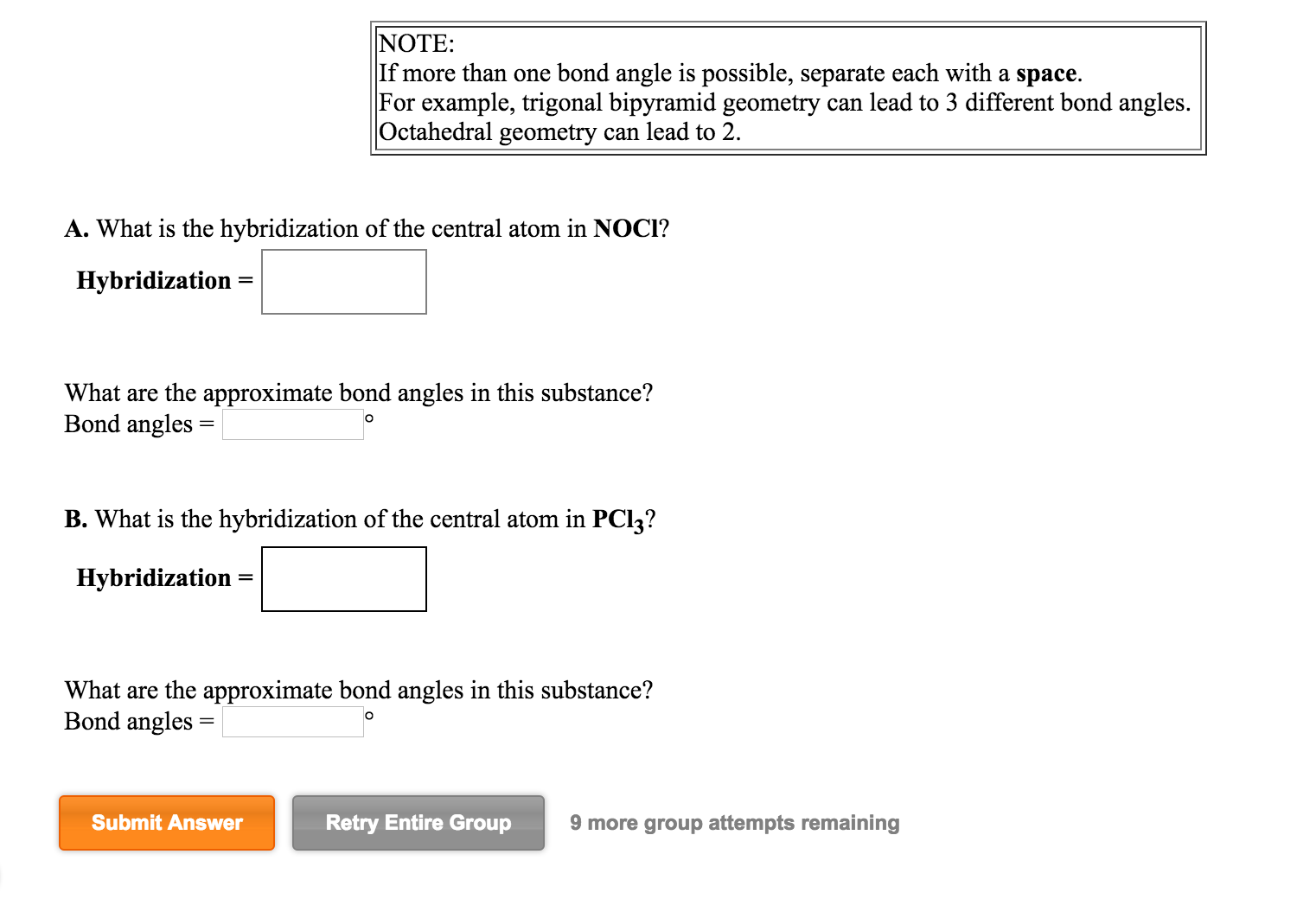
Solved What is the hybridization of the central atom in
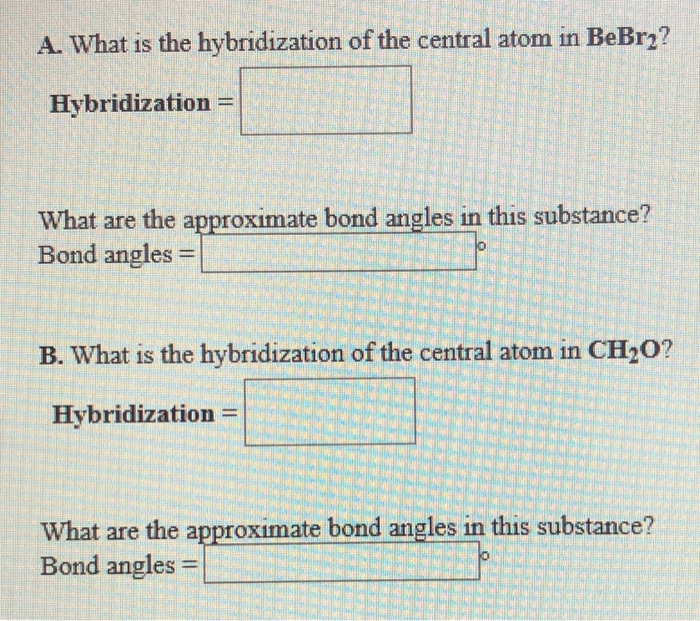
The hybridization of the central atom in so3 is sp2. Between each atom, we have double bonds. One is a sigma bond (σ), and the other one is the pi. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. This means that the sulfur atom has three hybrid.
Solved A. What is the hybridization of the central atom in
One is a sigma bond (σ), and the other one is the pi. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. As you can see in the structure; This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. The hybridization of.
SOLVED What is the hybridization of the central atom in GeH
This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. As you can see in the structure; The hybridization of the central atom in so3 is sp2. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. One is a sigma bond (σ),.
Solved A. What is the hybridization of the central atom in
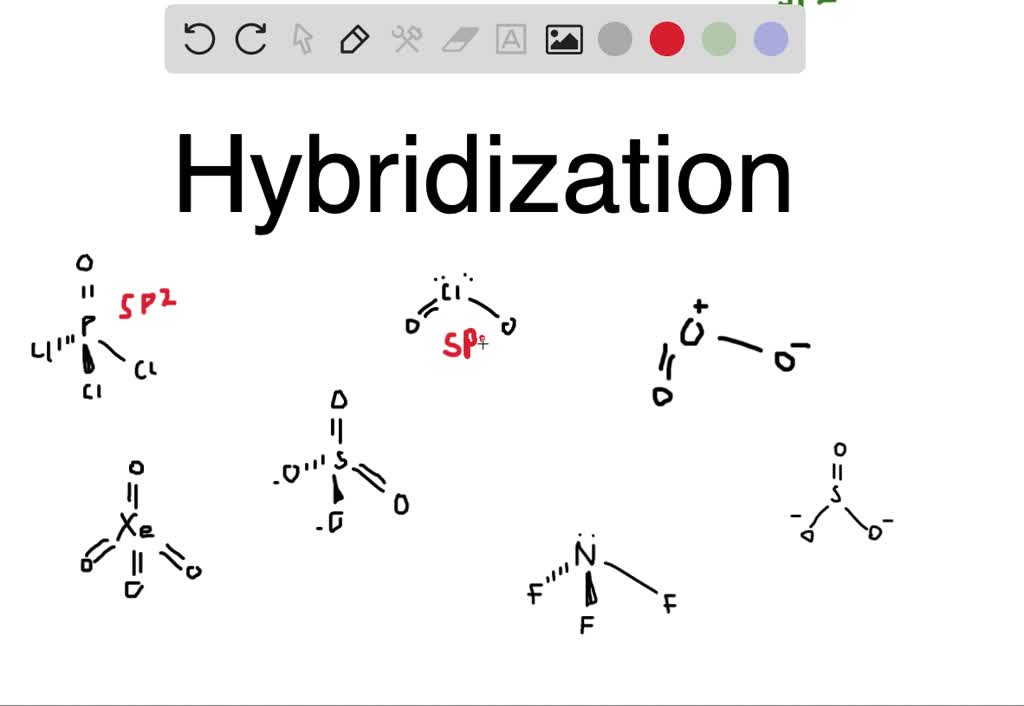
The hybridization of the central atom in so3 is sp2. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. As you can see in the structure; Between each atom, we have double.
Solved A. What is the hybridization of the central atom in
The hybridization of the central atom in so3 is sp2. As you can see in the structure; One is a sigma bond (σ), and the other one is the pi. Between each atom, we have double bonds. This means that the sulfur atom has three hybrid orbitals that are involved in bonding with.
Solved A. What is the hybridization of the central atom in
The hybridization of the central atom in so3 is sp2. Between each atom, we have double bonds. This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. As you can see in the.
SOLVED Give the expected hybridization of the central atom for the
As you can see in the structure; Between each atom, we have double bonds. In summary, hybridization of so3 refers to the process by which the sulfur atom in sulfur trioxide (so3) undergoes hybridization to. This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. The hybridization of the central atom in so3 is.
One Is A Sigma Bond (Σ), And The Other One Is The Pi.
As you can see in the structure; This means that the sulfur atom has three hybrid orbitals that are involved in bonding with. The hybridization of the central atom in so3 is sp2. Between each atom, we have double bonds.