What Is The Hybridization Of The Nitrogen Atoms In N2H4
What Is The Hybridization Of The Nitrogen Atoms In N2H4 - What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? B.) the nitrogen atoms in n2 are sp hybridized. This angle deviates from the ideal. N2h4 has a dipole moment of 1.85 d and is polar in nature. The hybridization of each nitrogen in the n2h4. The nitrogen atoms in n2h4. All of the nitrogen in the n2h4 molecule hybridizes to sp3. The hybridization of the nitrogen atoms in n2h4 is. The lewis structure for n2h4 is: (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat.
This angle deviates from the ideal. The hybridization of the nitrogen atoms in n2h4 is. The lewis structure for n2h4 is: The nitrogen atoms in n2h4. B.) the nitrogen atoms in n2 are sp hybridized. All of the nitrogen in the n2h4 molecule hybridizes to sp3. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat. The hybridization of each nitrogen in the n2h4. The total valence electron available for the n2h4 lewis structure is 14. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ?
The total valence electron available for the n2h4 lewis structure is 14. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat. This angle deviates from the ideal. The lewis structure for n2h4 is: The hybridization of each nitrogen in the n2h4. N2h4 has a dipole moment of 1.85 d and is polar in nature. The nitrogen atoms in n2h4. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? The hybridization of the nitrogen atoms in n2h4 is. B.) the nitrogen atoms in n2 are sp hybridized.
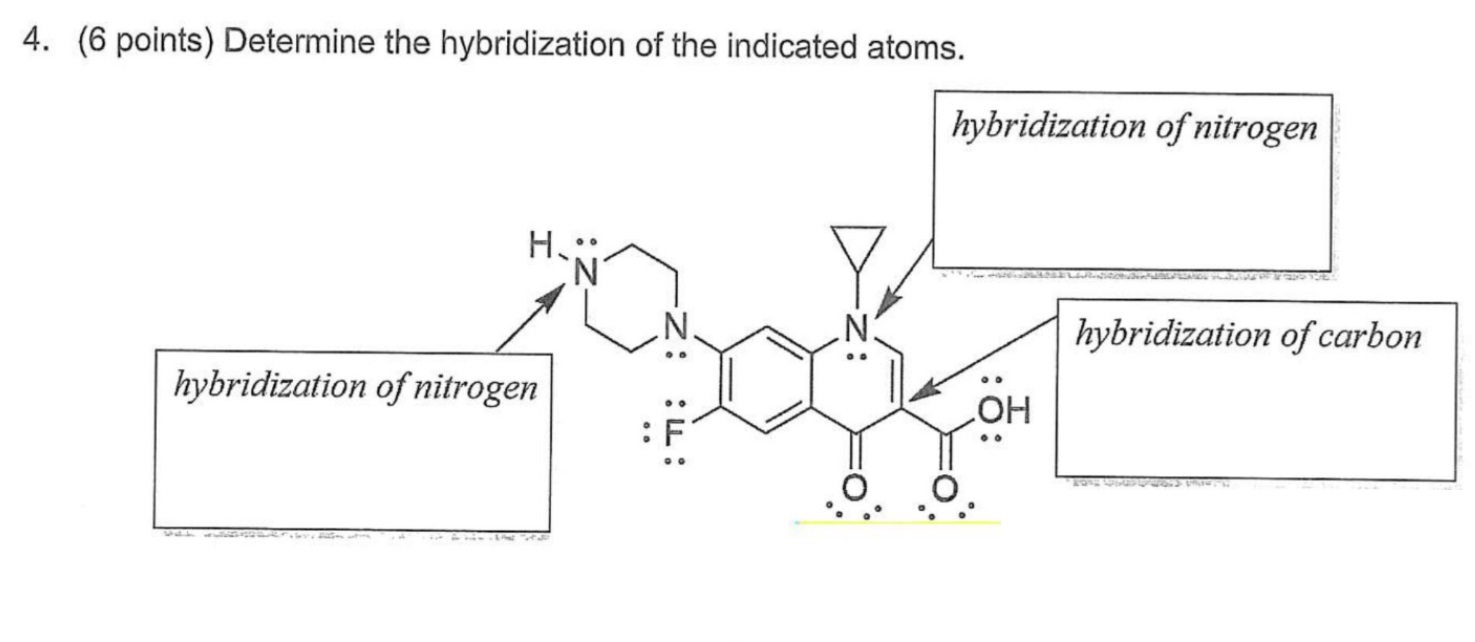
Solved 4. (6 points) Determine the hybridization of the
The hybridization of each nitrogen in the n2h4. This angle deviates from the ideal. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? All of the nitrogen in the n2h4 molecule hybridizes to sp3. B.) the nitrogen atoms in n2 are sp hybridized.
Hybridization Definition, Types, Rules, Examples, 58 OFF
The hybridization of the nitrogen atoms in n2h4 is. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? N2h4 has a dipole moment of 1.85 d and is polar in nature. All of the nitrogen in the n2h4 molecule hybridizes to sp3. The hybridization of each nitrogen in the n2h4.
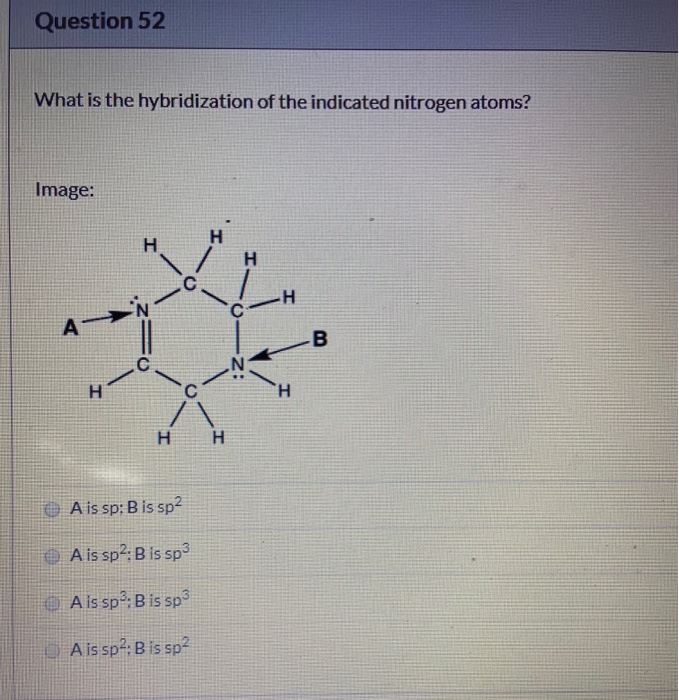
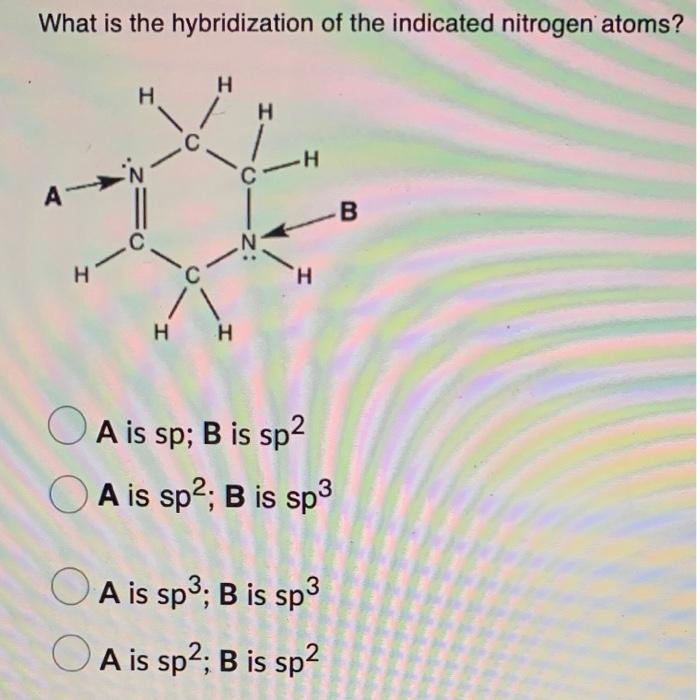
Solved What is the hybridization of the indicated nitrogen
N2h4 has a dipole moment of 1.85 d and is polar in nature. The total valence electron available for the n2h4 lewis structure is 14. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat. The hybridization of the nitrogen atoms in n2h4 is. The nitrogen atoms in n2h4.
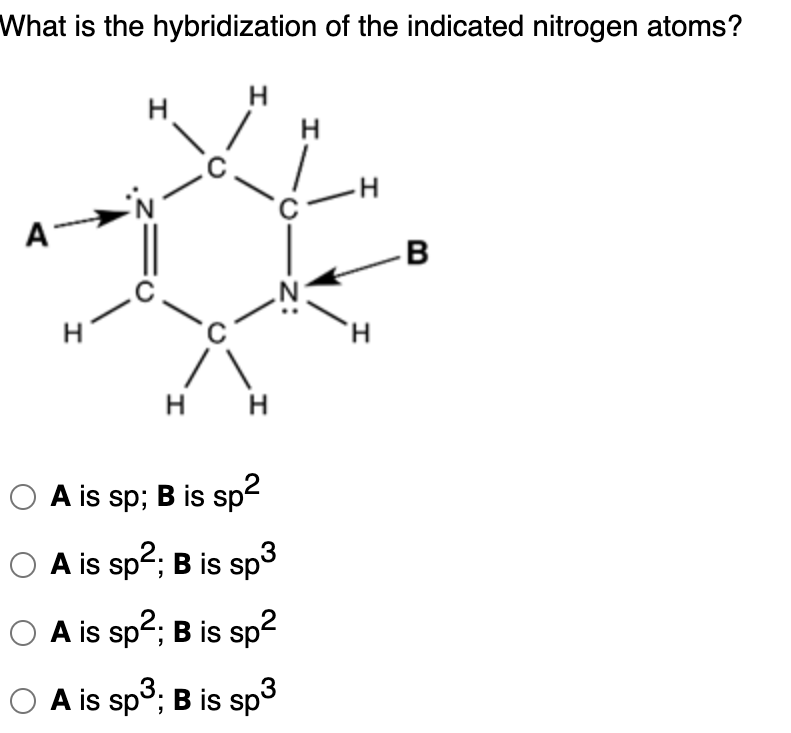
Solved What is the hybridization of the indicated nitrogen
What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? All of the nitrogen in the n2h4 molecule hybridizes to sp3. B.) the nitrogen atoms in n2 are sp hybridized. This angle deviates from the ideal. The hybridization of each nitrogen in the n2h4.
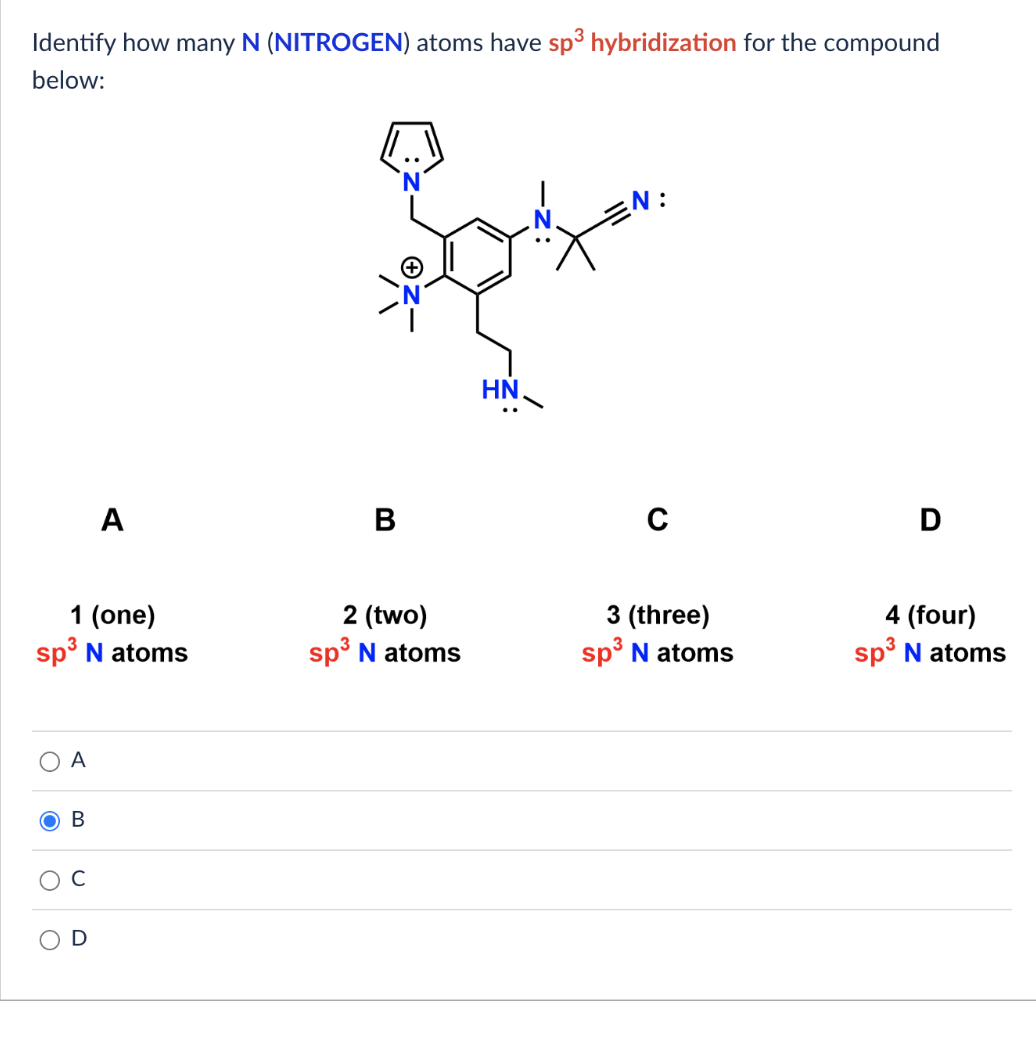
[Solved] Identify how many N ( NITROGEN ) atoms have sp 3 hybridization
All of the nitrogen in the n2h4 molecule hybridizes to sp3. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat. The hybridization of each nitrogen in the n2h4. The nitrogen atoms in n2h4. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ?
[Solved] Identify how many N (NITROGEN) atoms have sp2
This angle deviates from the ideal. The nitrogen atoms in n2h4. The hybridization of each nitrogen in the n2h4. N2h4 has a dipole moment of 1.85 d and is polar in nature. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ?
Solved Question 52 What Is The Hybridization Of The Indic...
The hybridization of the nitrogen atoms in n2h4 is. All of the nitrogen in the n2h4 molecule hybridizes to sp3. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? The lewis structure for n2h4 is: The nitrogen atoms in n2h4.
Solved Hybridization of nitrogen atom in N2H4 is. is whereas
The hybridization of each nitrogen in the n2h4. All of the nitrogen in the n2h4 molecule hybridizes to sp3. The nitrogen atoms in n2h4. The total valence electron available for the n2h4 lewis structure is 14. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ?
Solved Identify how many N (NITROGEN) atoms have \\(
B.) the nitrogen atoms in n2 are sp hybridized. The hybridization of the nitrogen atoms in n2h4 is. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? N2h4 has a dipole moment of 1.85 d and is.
[Solved] Identify how many N (NITROGEN) atoms have sp hybridization for
N2h4 has a dipole moment of 1.85 d and is polar in nature. B.) the nitrogen atoms in n2 are sp hybridized. The lewis structure for n2h4 is: This angle deviates from the ideal. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat.
The Total Valence Electron Available For The N2H4 Lewis Structure Is 14.
The lewis structure for n2h4 is: The hybridization of each nitrogen in the n2h4. All of the nitrogen in the n2h4 molecule hybridizes to sp3. (a) determine the hybridization of each n atom in ibrutinib, a drug used to treat.
N2H4 Has A Dipole Moment Of 1.85 D And Is Polar In Nature.
B.) the nitrogen atoms in n2 are sp hybridized. The nitrogen atoms in n2h4. What is the hybridization of n atoms in n 2 {_2} 2 h 4 {_4} 4 ? The hybridization of the nitrogen atoms in n2h4 is.
![[Solved] Identify how many N (NITROGEN) atoms have sp2](https://media.cheggcdn.com/media/250/25001471-53e2-40e1-97ef-668977575fc1/phppbkvdZ)