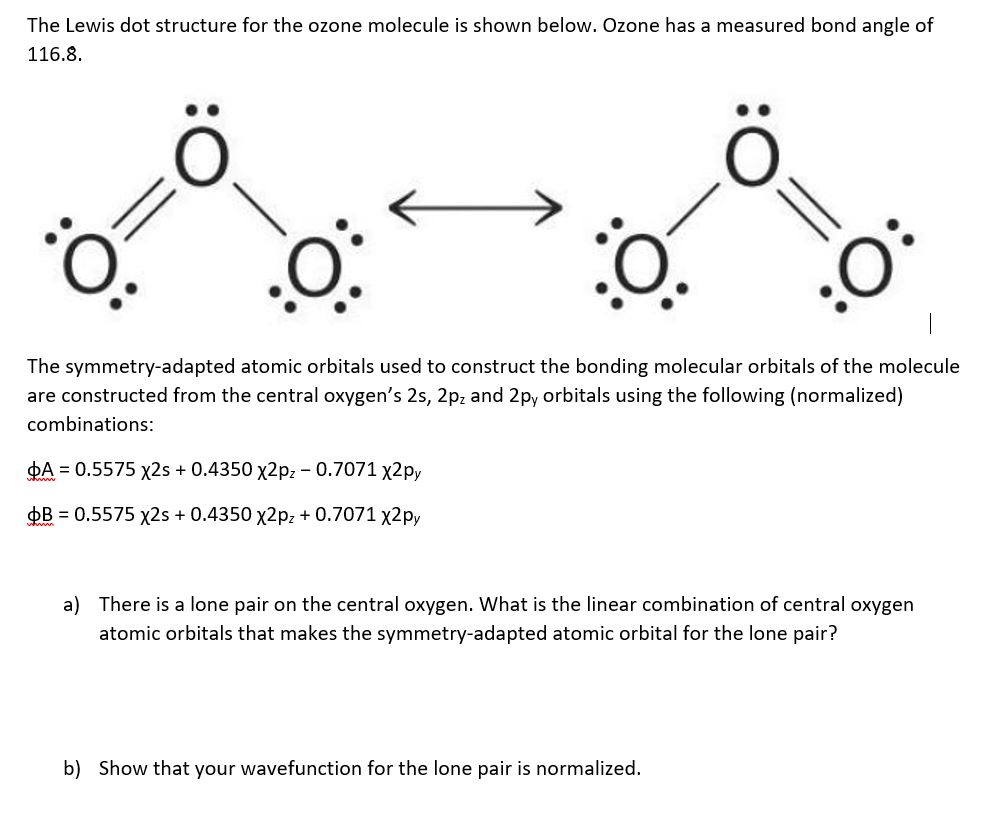
What Is The Lewis Structure For O3
What Is The Lewis Structure For O3 - The octet rule states that there should be eight electrons in the outer shell or orbit of. What is the lewis structure of ozone (o3)? Lewis structure is based on the octet rule. In the lewis structure of ozone, there are one double boond and one. The typical lewis structure of ozone depicts formal charge separation. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms.
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The typical lewis structure of ozone depicts formal charge separation. In the lewis structure of ozone, there are one double boond and one. Lewis structure is based on the octet rule. The octet rule states that there should be eight electrons in the outer shell or orbit of. What is the lewis structure of ozone (o3)?
In the lewis structure of ozone, there are one double boond and one. The octet rule states that there should be eight electrons in the outer shell or orbit of. The typical lewis structure of ozone depicts formal charge separation. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Lewis structure is based on the octet rule. What is the lewis structure of ozone (o3)?
Lewis Structure
The octet rule states that there should be eight electrons in the outer shell or orbit of. What is the lewis structure of ozone (o3)? The typical lewis structure of ozone depicts formal charge separation. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double boond and one.
Draw The Lewis Structure Of Ozone O3
In the lewis structure of ozone, there are one double boond and one. The octet rule states that there should be eight electrons in the outer shell or orbit of. The typical lewis structure of ozone depicts formal charge separation. What is the lewis structure of ozone (o3)? Lewis structure is based on the octet rule.
O3 Lewis Structure Drawings, Hybridization, Shape, Charges, Pair, And
Lewis structure is based on the octet rule. The octet rule states that there should be eight electrons in the outer shell or orbit of. In the lewis structure of ozone, there are one double boond and one. What is the lewis structure of ozone (o3)? Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms.
O3 Lewis Structure, Polarity, Hybridization, Shape and Much More
What is the lewis structure of ozone (o3)? The typical lewis structure of ozone depicts formal charge separation. The octet rule states that there should be eight electrons in the outer shell or orbit of. Lewis structure is based on the octet rule. In the lewis structure of ozone, there are one double boond and one.
12+ O3 Lewis Structure Robhosking Diagram
The octet rule states that there should be eight electrons in the outer shell or orbit of. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. What is the lewis structure of ozone (o3)? In the lewis structure of ozone, there are one double boond and one. Lewis structure is based on the octet rule.
Ozone Molecule Lewis Structure
The octet rule states that there should be eight electrons in the outer shell or orbit of. In the lewis structure of ozone, there are one double boond and one. What is the lewis structure of ozone (o3)? The typical lewis structure of ozone depicts formal charge separation. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms.
Draw The Lewis Structure Of Ozone O3
The typical lewis structure of ozone depicts formal charge separation. Lewis structure is based on the octet rule. What is the lewis structure of ozone (o3)? In the lewis structure of ozone, there are one double boond and one. The octet rule states that there should be eight electrons in the outer shell or orbit of.
O3 Lewis Structure Drawings, Hybridization, Shape, Charges, Pair, And
In the lewis structure of ozone, there are one double boond and one. The typical lewis structure of ozone depicts formal charge separation. What is the lewis structure of ozone (o3)? Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Lewis structure is based on the octet rule.
O3 Lewis Structure (Ozone) Chemistry, Lewis, Ozone
Lewis structure is based on the octet rule. The typical lewis structure of ozone depicts formal charge separation. In the lewis structure of ozone, there are one double boond and one. The octet rule states that there should be eight electrons in the outer shell or orbit of. What is the lewis structure of ozone (o3)?
O3 Lewis Structure Drawings, Hybridization, Shape, Charges, Pair, And
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Lewis structure is based on the octet rule. In the lewis structure of ozone, there are one double boond and one. The octet rule states that there should be eight electrons in the outer shell or orbit of. The typical lewis structure of ozone depicts formal charge separation.
Lewis Structure Is Based On The Octet Rule.
What is the lewis structure of ozone (o3)? The typical lewis structure of ozone depicts formal charge separation. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The octet rule states that there should be eight electrons in the outer shell or orbit of.