What Is The Molecular Shape Of Pcl3
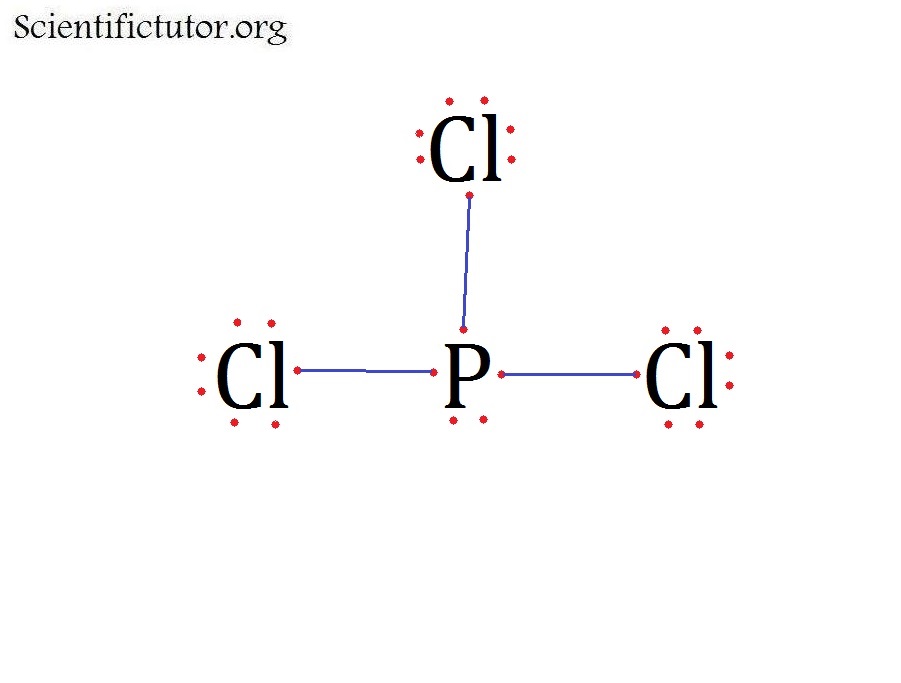
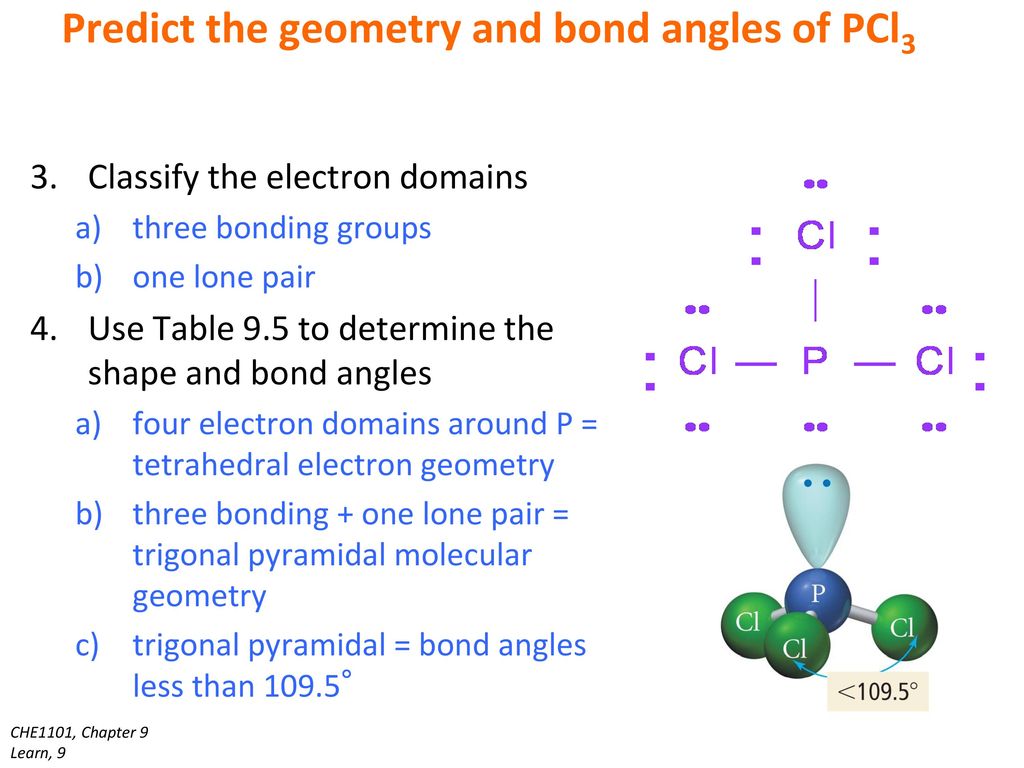
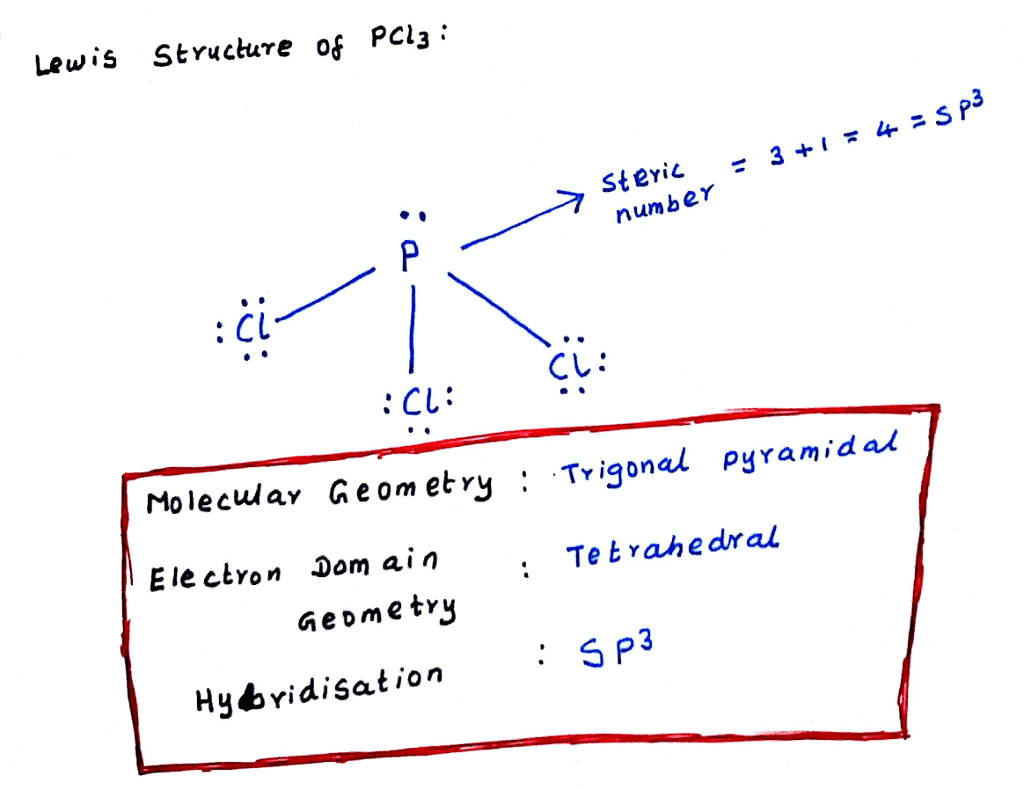
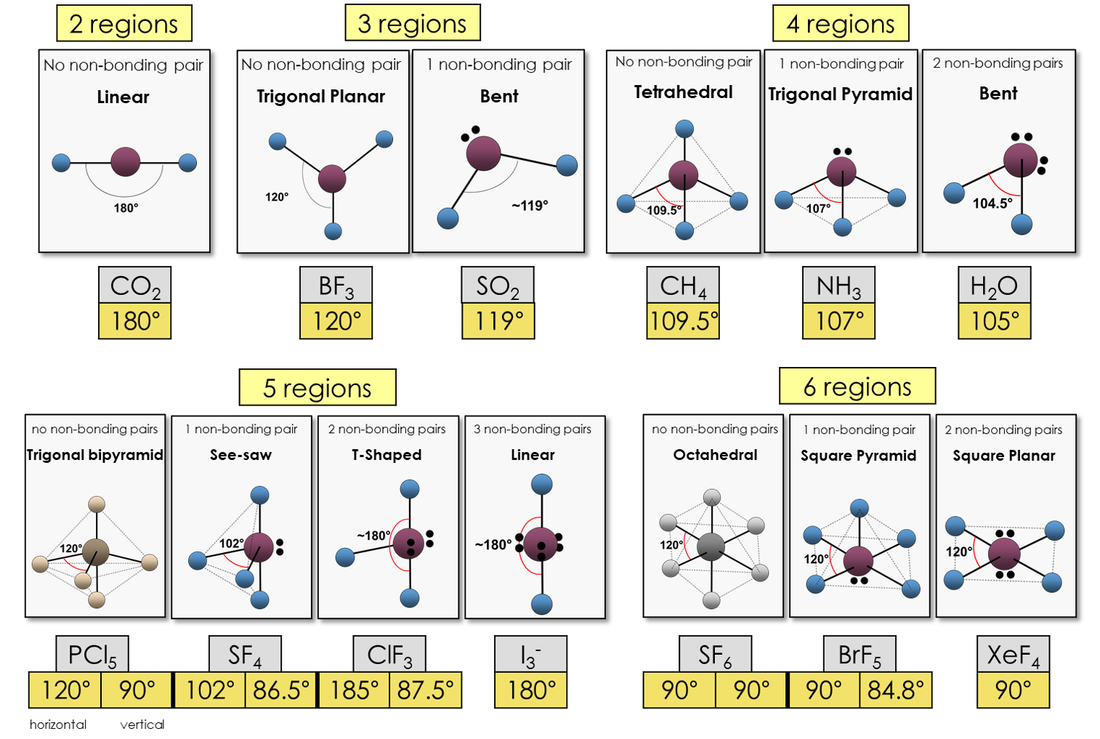
What Is The Molecular Shape Of Pcl3 - The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The lewis structure of #pcl_3# is the following: This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal.
The central p atom has one lone pair of electrons and three bond pairs of electrons. The lewis structure of #pcl_3# is the following: This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The shape of a p c l 3 molecule is trigonal pyramidal.
The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The lewis structure of #pcl_3# is the following:
Chem Molecular Shape (Molecular Geometry) Scientific Tutor
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. This is under the form of #ax_3e# where #x#.
SOLVED Draw the Lewis structure for each of the following and then
The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This is under the form of #ax_3e# where #x#.
PCl3 Lewis Structure, Molecular Geometry, Bond Angle,, 43 OFF
This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l.
PCl3 Lewis Structure, Molecular Geometry, Bond Angle,, 43 OFF
The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This.
Pcl3 Lewis Structure Molecular Geometry
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and.
4. Molecular Shapes
The lewis structure of #pcl_3# is the following: This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal. The molecular geometry or shape of pcl 3 is a.
Arsenic Trichloride 3d Balls Pcl3 Molecular Shape, HD Png Download vhv
The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. This.
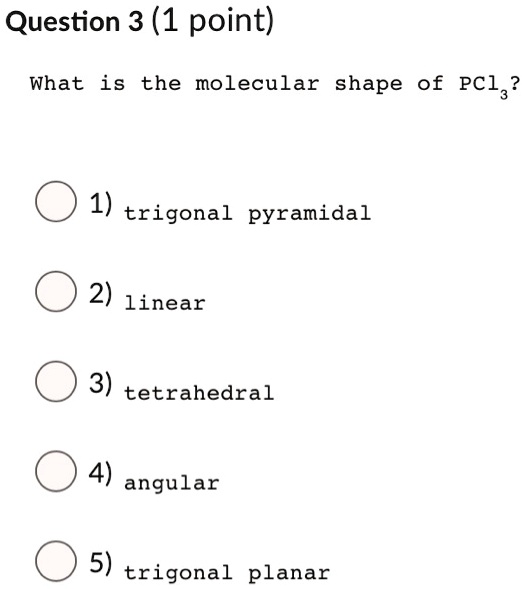
SOLVED Question 3 (1 point) What is the molecular shape of PCl3 1
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal. The lewis structure of #pcl_3# is the following: This.
PCl3 Molecular Geometry Science Education and Tutorials
The central p atom has one lone pair of electrons and three bond pairs of electrons. The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. This is under the form of #ax_3e# where #x# represents the bounded groups #cl#. The molecular geometry or shape of pcl 3 is a.
SOLVED NH3, has the same molecular shape as PCl3. Which intermolecular
The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The lewis structure of #pcl_3# is the following: The shape of a p c l 3 molecule is trigonal pyramidal. The central p atom has one lone pair of electrons and three bond pairs of electrons. This.
This Is Under The Form Of #Ax_3E# Where #X# Represents The Bounded Groups #Cl#.
The lewis structure of #pcl_3# is the following: The molecular geometry or shape of pcl 3 is a trigonal pyramid, because, the lone pair present on the central phosphorous (p) atom. The central p atom has one lone pair of electrons and three bond pairs of electrons. The shape of a p c l 3 molecule is trigonal pyramidal.