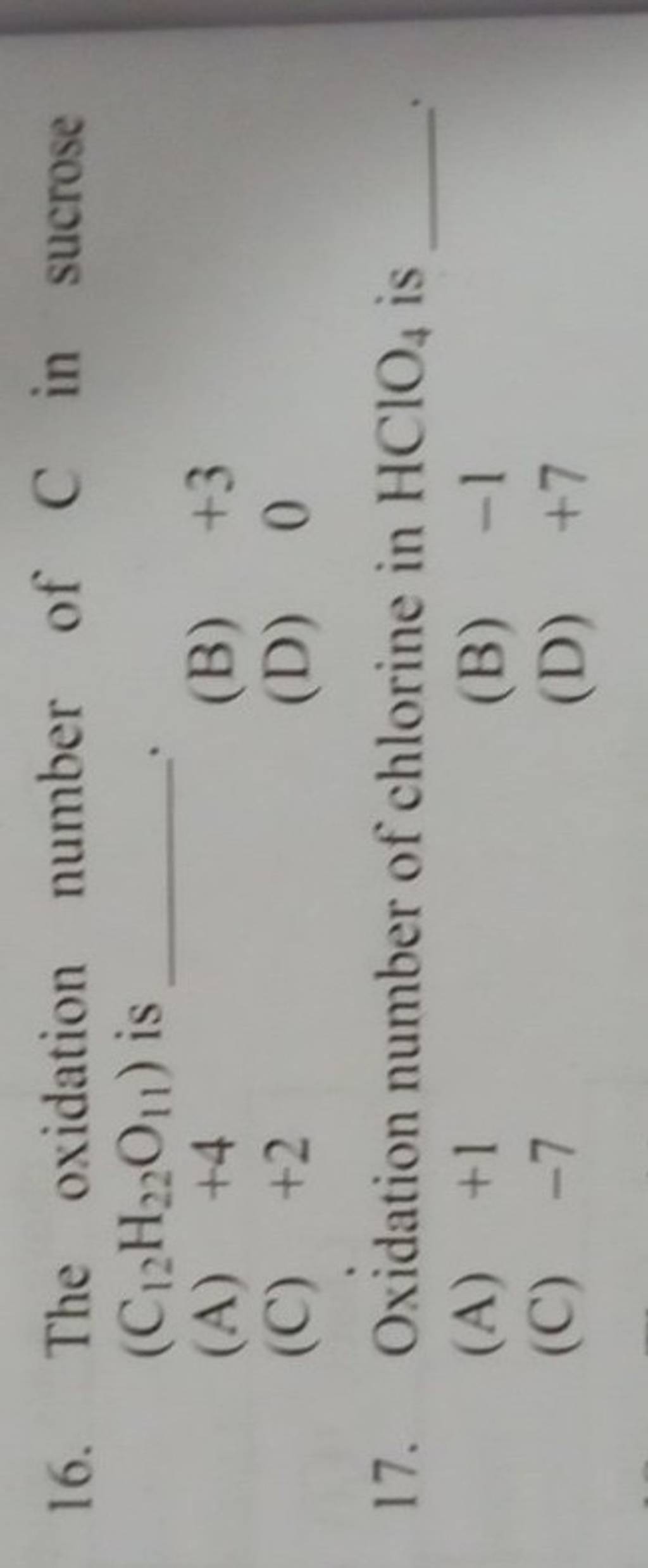What Is The Oxidation Number Of Chlorine In Clo4
What Is The Oxidation Number Of Chlorine In Clo4 - The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). To determine the oxidation number, we. There are 2 steps to solve this one. The oxidation number for chlorine can vary depending on the compound it is part of. The oxidation number of chlorine in clo4 is +7.
The oxidation number for chlorine can vary depending on the compound it is part of. There are 2 steps to solve this one. The oxidation number of chlorine in clo4 is +7. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). To determine the oxidation number, we.
The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). The oxidation number for chlorine can vary depending on the compound it is part of. The oxidation number of chlorine in clo4 is +7. To determine the oxidation number, we. There are 2 steps to solve this one.
Solved 5. Find the oxidation number of chlorine in each of
To determine the oxidation number, we. The oxidation number of chlorine in clo4 is +7. There are 2 steps to solve this one. The oxidation number for chlorine can vary depending on the compound it is part of. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion).
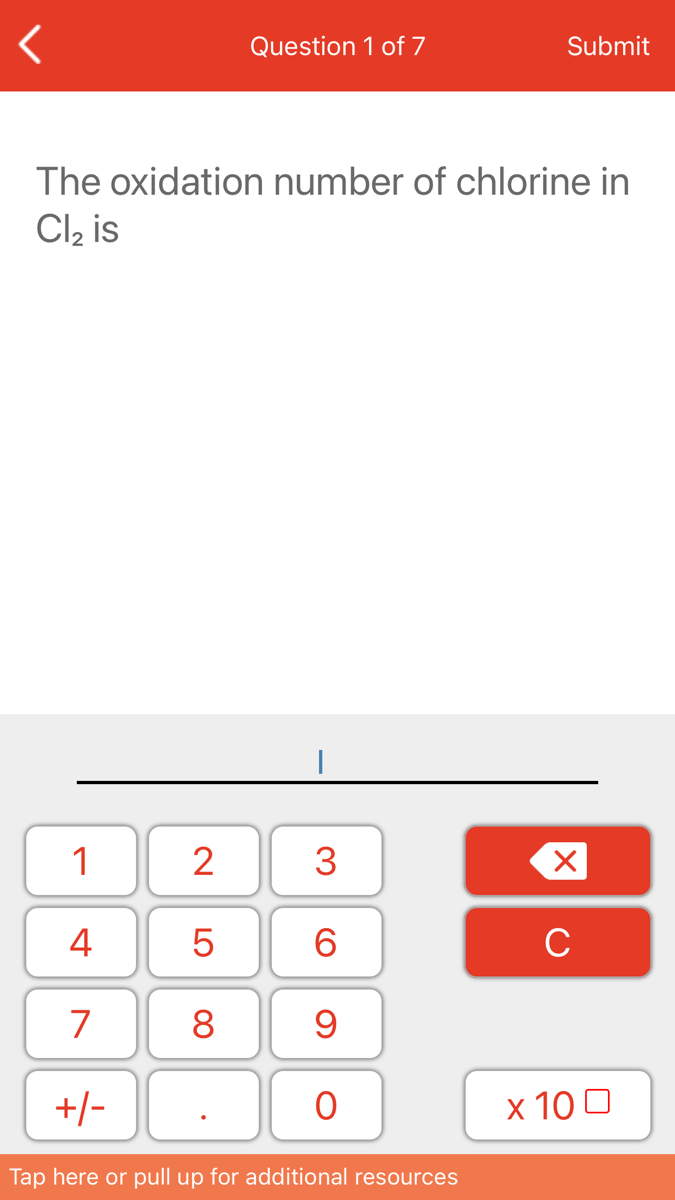
Answered The oxidation number of chlorine in Cl2… bartleby
The oxidation number of chlorine in clo4 is +7. To determine the oxidation number, we. There are 2 steps to solve this one. The oxidation number for chlorine can vary depending on the compound it is part of. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion).
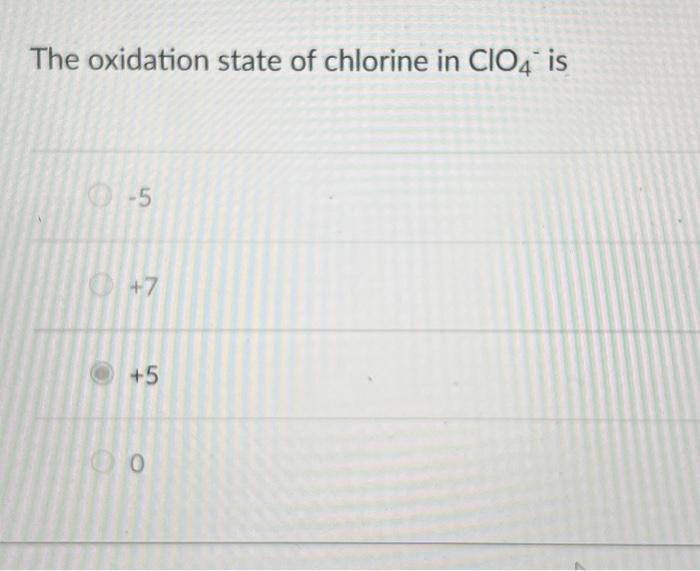
Solved The oxidation state of chlorine in ClO4−is −5 +7 +5 0
The oxidation number for chlorine can vary depending on the compound it is part of. There are 2 steps to solve this one. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). To determine the oxidation number, we. The oxidation number of chlorine in clo4 is +7.
SOLVEDRank the following oxoanions in order of decreasing oxidation
To determine the oxidation number, we. The oxidation number of chlorine in clo4 is +7. There are 2 steps to solve this one. The oxidation number for chlorine can vary depending on the compound it is part of. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion).
Oxidation Number Rules Chart
The oxidation number of chlorine in clo4 is +7. The oxidation number for chlorine can vary depending on the compound it is part of. To determine the oxidation number, we. There are 2 steps to solve this one. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion).
SOLVED In which one of the following molecules does chlorine have the
The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). There are 2 steps to solve this one. The oxidation number of chlorine in clo4 is +7. The oxidation number for chlorine can vary depending on the compound it is part of. To determine the oxidation number, we.
Oxidation number of chlorine atoms in CaOCl2 are Filo
The oxidation number for chlorine can vary depending on the compound it is part of. There are 2 steps to solve this one. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). To determine the oxidation number, we. The oxidation number of chlorine in clo4 is +7.
Oxidation number of chlorine in HClO4 is Filo
The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). There are 2 steps to solve this one. The oxidation number of chlorine in clo4 is +7. To determine the oxidation number, we. The oxidation number for chlorine can vary depending on the compound it is part of.
Oxidation Number of Chlorine LinatuSims
The oxidation number of chlorine in clo4 is +7. To determine the oxidation number, we. The oxidation number for chlorine can vary depending on the compound it is part of. There are 2 steps to solve this one. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion).
Solved What is the oxidation number of chlorine in HClO4 ?
The oxidation number for chlorine can vary depending on the compound it is part of. There are 2 steps to solve this one. To determine the oxidation number, we. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). The oxidation number of chlorine in clo4 is +7.
To Determine The Oxidation Number, We.
There are 2 steps to solve this one. The oxidation number for chlorine can vary depending on the compound it is part of. The question is asking for the oxidation number of chlorine (cl) in the compound clo4 (perchlorate ion). The oxidation number of chlorine in clo4 is +7.