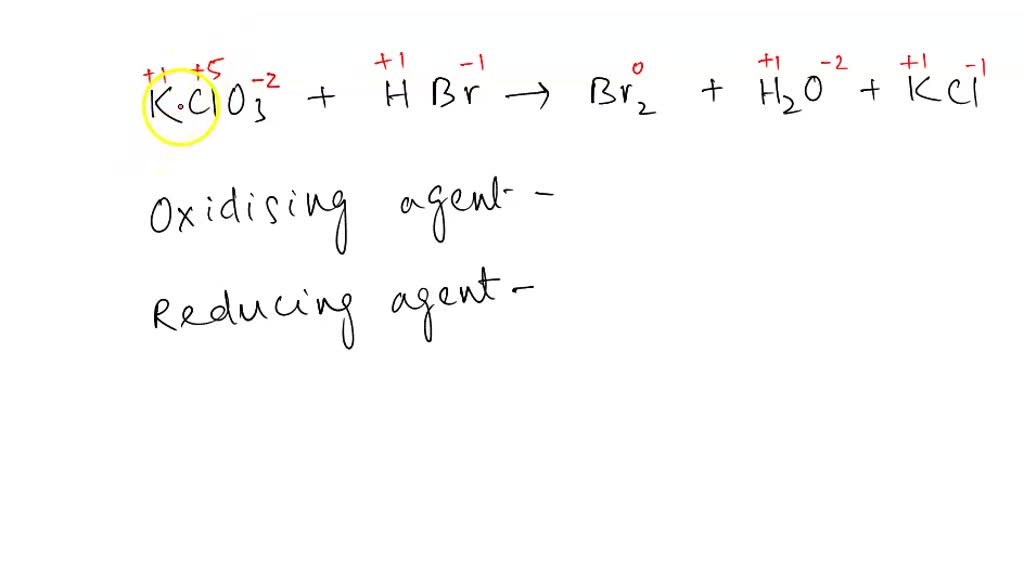What Is The Oxidation Number Of Chlorine In Kclo3
What Is The Oxidation Number Of Chlorine In Kclo3 - $$ \textbf{explanation} $$ for the chlorate, we. And the oxidation number of chlorine in potassium. The oxidation number of chlorine in kclo3 is +5 (option a). The oxidation number of an element is. The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. The oxidation number of chlorine in kclo3 is +5. Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$. How to calculate oxidation number?
$$ \textbf{explanation} $$ for the chlorate, we. How to calculate oxidation number? The oxidation number of chlorine in kclo3 is +5. The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. The oxidation number of chlorine in kclo3 is +5 (option a). And the oxidation number of chlorine in potassium. The oxidation number of an element is. Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$.
$$ \textbf{explanation} $$ for the chlorate, we. How to calculate oxidation number? The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. The oxidation number of an element is. And the oxidation number of chlorine in potassium. The oxidation number of chlorine in kclo3 is +5. The oxidation number of chlorine in kclo3 is +5 (option a). Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$.
Oxidation number of chlorine atoms in CaOCl2 are Filo
How to calculate oxidation number? $$ \textbf{explanation} $$ for the chlorate, we. The oxidation number of chlorine in kclo3 is +5 (option a). The oxidation number of chlorine in kclo3 is +5. The oxidation number of chlorine in potassium chlorate, kclo_3, is +v.
Oxidation number of Cl in KClO3
The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$. The oxidation number of chlorine in kclo3 is +5 (option a). $$ \textbf{explanation} $$ for the chlorate, we. The oxidation number of an element is.
Oxidation Number of Chlorine LinatuSims
The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. The oxidation number of an element is. Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$. The oxidation number of chlorine in kclo3 is +5. And the oxidation number of chlorine in potassium.
What Is Chlorine's Oxidation Number
The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. $$ \textbf{explanation} $$ for the chlorate, we. The oxidation number of chlorine in kclo3 is +5 (option a). How to calculate oxidation number? Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$.
Solved 5. Find the oxidation number of chlorine in each of
How to calculate oxidation number? Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$. $$ \textbf{explanation} $$ for the chlorate, we. The oxidation number of chlorine in kclo3 is +5 (option a). The oxidation number of an element is.
What Is Chlorine's Oxidation Number
The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. How to calculate oxidation number? The oxidation number of chlorine in kclo3 is +5 (option a). The oxidation number of an element is. The oxidation number of chlorine in kclo3 is +5.
Solved For The Following Reaction KClO, → KCl + O, Assign...
And the oxidation number of chlorine in potassium. How to calculate oxidation number? The oxidation number of an element is. Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$. The oxidation number of chlorine in potassium chlorate, kclo_3, is +v.
Oxidation number of Cl in KClO3
And the oxidation number of chlorine in potassium. The oxidation number of an element is. How to calculate oxidation number? The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. The oxidation number of chlorine in kclo3 is +5 (option a).
SOLVED Assign oxidation numbers to all atoms in the equation. Mention
The oxidation number of chlorine in kclo3 is +5. The oxidation number of an element is. Thee oxidation number of chlorine in potassium chlorate, $$ kclo_{3}, $$ is $$ +v $$. And the oxidation number of chlorine in potassium. The oxidation number of chlorine in potassium chlorate, kclo_3, is +v.
Oxidation Number of Chlorine LinatuSims
$$ \textbf{explanation} $$ for the chlorate, we. And the oxidation number of chlorine in potassium. The oxidation number of chlorine in potassium chlorate, kclo_3, is +v. The oxidation number of chlorine in kclo3 is +5 (option a). How to calculate oxidation number?
The Oxidation Number Of Chlorine In Potassium Chlorate, Kclo_3, Is +V.
And the oxidation number of chlorine in potassium. The oxidation number of chlorine in kclo3 is +5. The oxidation number of chlorine in kclo3 is +5 (option a). The oxidation number of an element is.
Thee Oxidation Number Of Chlorine In Potassium Chlorate, $$ Kclo_{3}, $$ Is $$ +V $$.
$$ \textbf{explanation} $$ for the chlorate, we. How to calculate oxidation number?