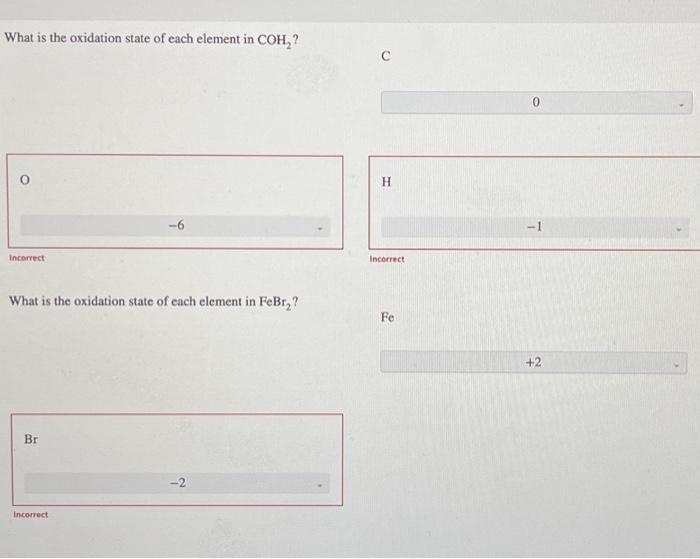
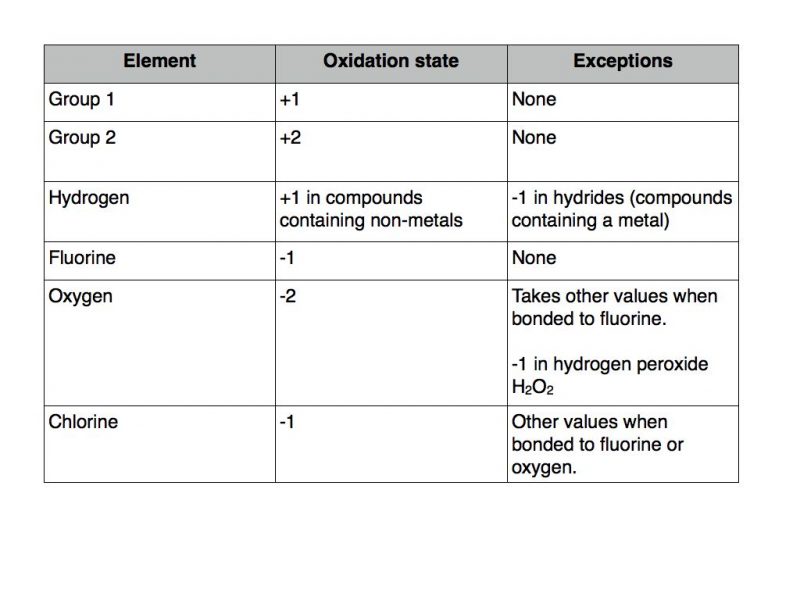
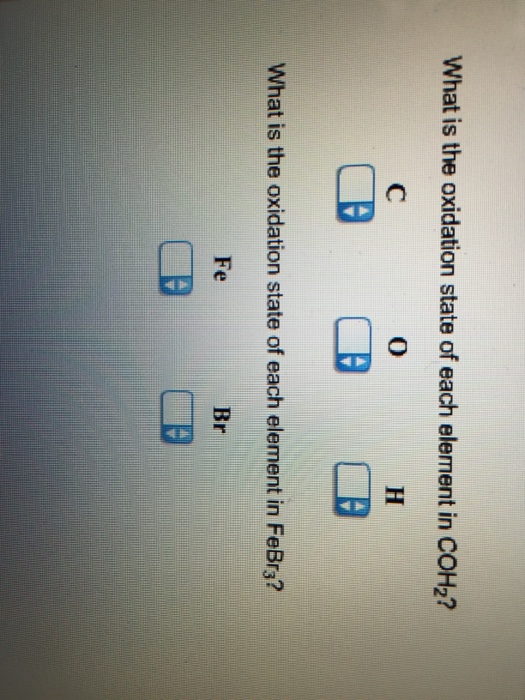
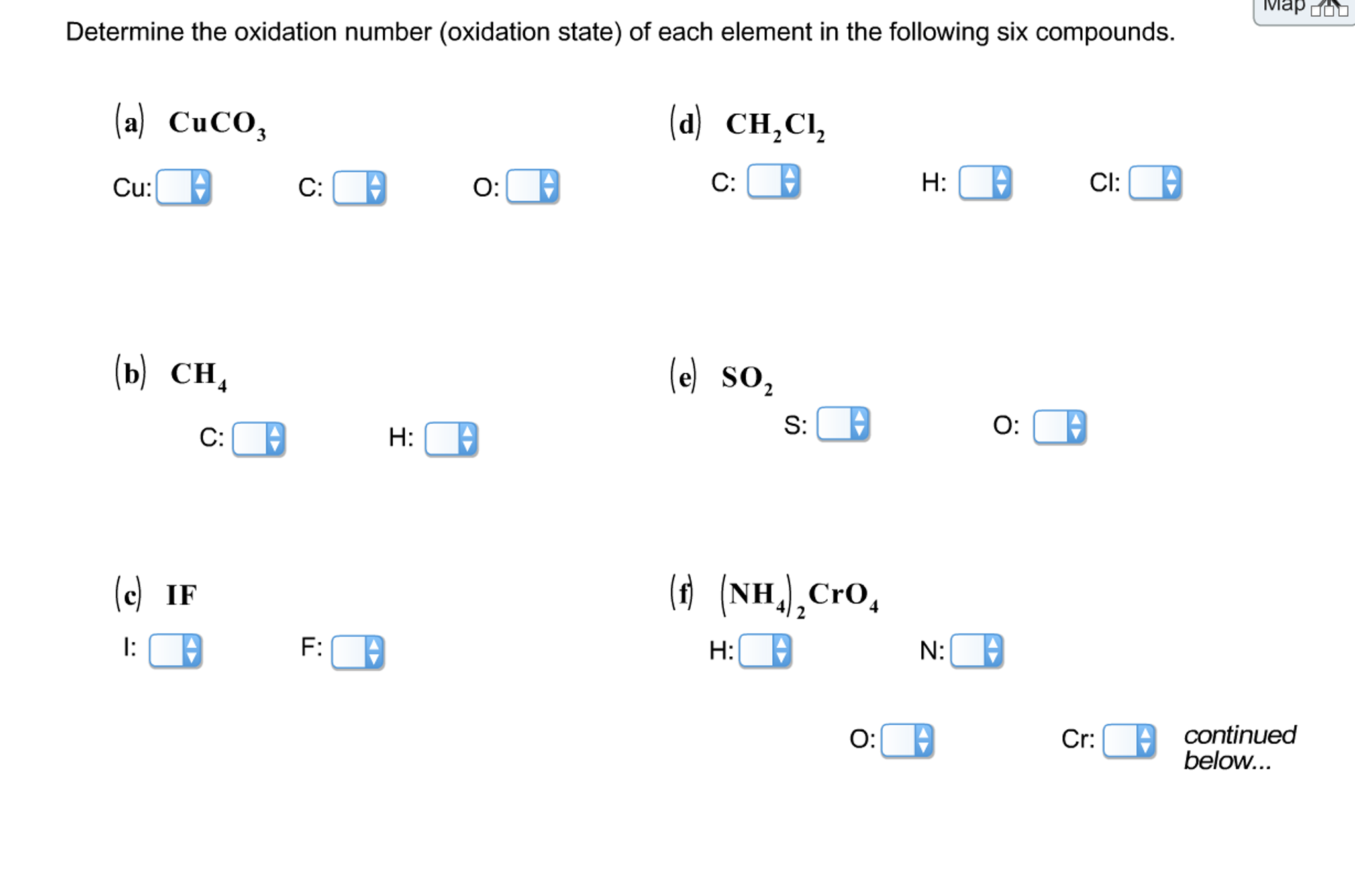
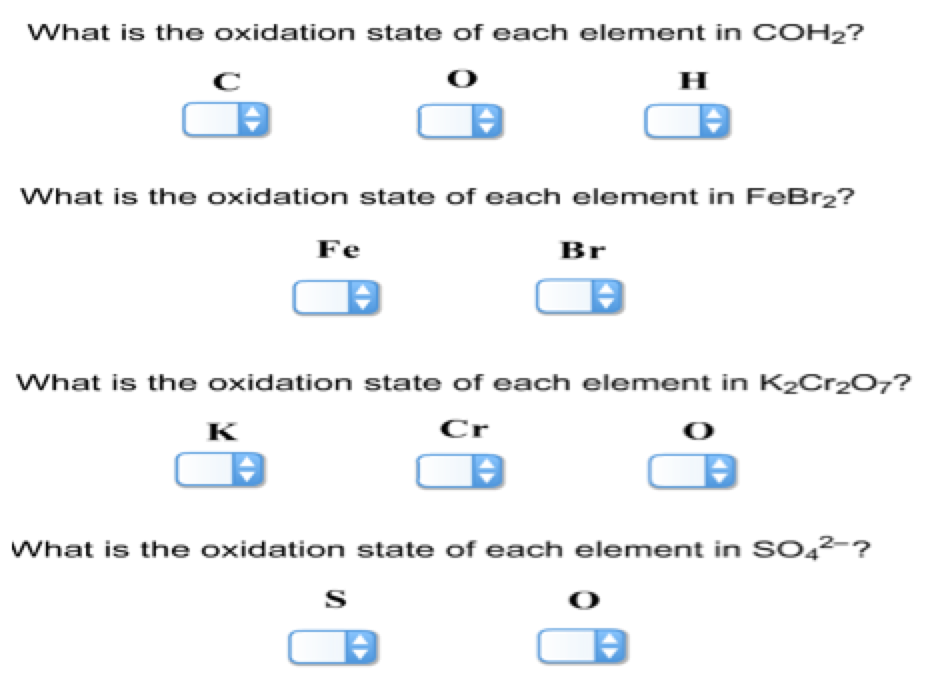
What Is The Oxidation State Of Each Element In Coh2
What Is The Oxidation State Of Each Element In Coh2 - In coh2, the oxidation state (or. Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. The overall oxidation state of the molecule is zero,. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to.
In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to. In coh2, the oxidation state (or. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. The overall oxidation state of the molecule is zero,.
In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to. Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. In coh2, the oxidation state (or. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. The overall oxidation state of the molecule is zero,.
Solved What is the oxidation state of each element in COH2 ?
In coh2, the oxidation state (or. Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. The overall oxidation state of the molecule is zero,. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Conceptually, the oxidation state, which may be positive, negative or zero, is.
Oxidation state examples Online Chemistry Tutor
Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. The overall oxidation state of the molecule is zero,. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom.
Solved What is the oxidation state of each element in COH2?
Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. In coh2, the oxidation state (or. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to. The overall oxidation state of the molecule is zero,. Start by assigning formal oxidation states to the most.
Solved What is the oxidation state of each element in COH,
In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. In coh2, the oxidation state (or. The overall oxidation state of the molecule is zero,. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to..
Solved Determine the oxidation number (oxidation state) of
In coh2, the oxidation state (or. The overall oxidation state of the molecule is zero,. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. Next.
In 3d series which element shows highest oxidation state Filo
Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to. In coh2, the.
Solved What is the oxidation state of each element in COH2 ?
Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. In coh2, the oxidation state (or. The overall oxidation state of the molecule is zero,. Conceptually, the oxidation state, which may be positive, negative or zero, is the.
Solved What is the oxidation state of each element in COH2?
Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. The overall oxidation state of the molecule is zero,. In coh2, the oxidation state (or. Next.
Solved What is the oxidation state of each element in COH_2?
The overall oxidation state of the molecule is zero,. In coh2, the oxidation state (or. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. Next is hydrogen (h),.
Determine the highest possible oxidation state for each element. a. V b
In coh2, the oxidation state (or. The overall oxidation state of the molecule is zero,. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. Conceptually, the oxidation state,.
Conceptually, The Oxidation State, Which May Be Positive, Negative Or Zero, Is The Hypothetical Charge That An Atom Would Have If All Bonds To.
In coh2, the oxidation state (or. Start by assigning formal oxidation states to the most electronegative elements, then discover the positive limits of the least electronegative. Next is hydrogen (h), which has a preferred oxidation state of $$+1$$. In this article, we will delve into the intricacies of coh2 and explore the oxidation state of carbon, oxygen, and hydrogen.