What Is The Valence Electron Configuration For The Sulfur Atom
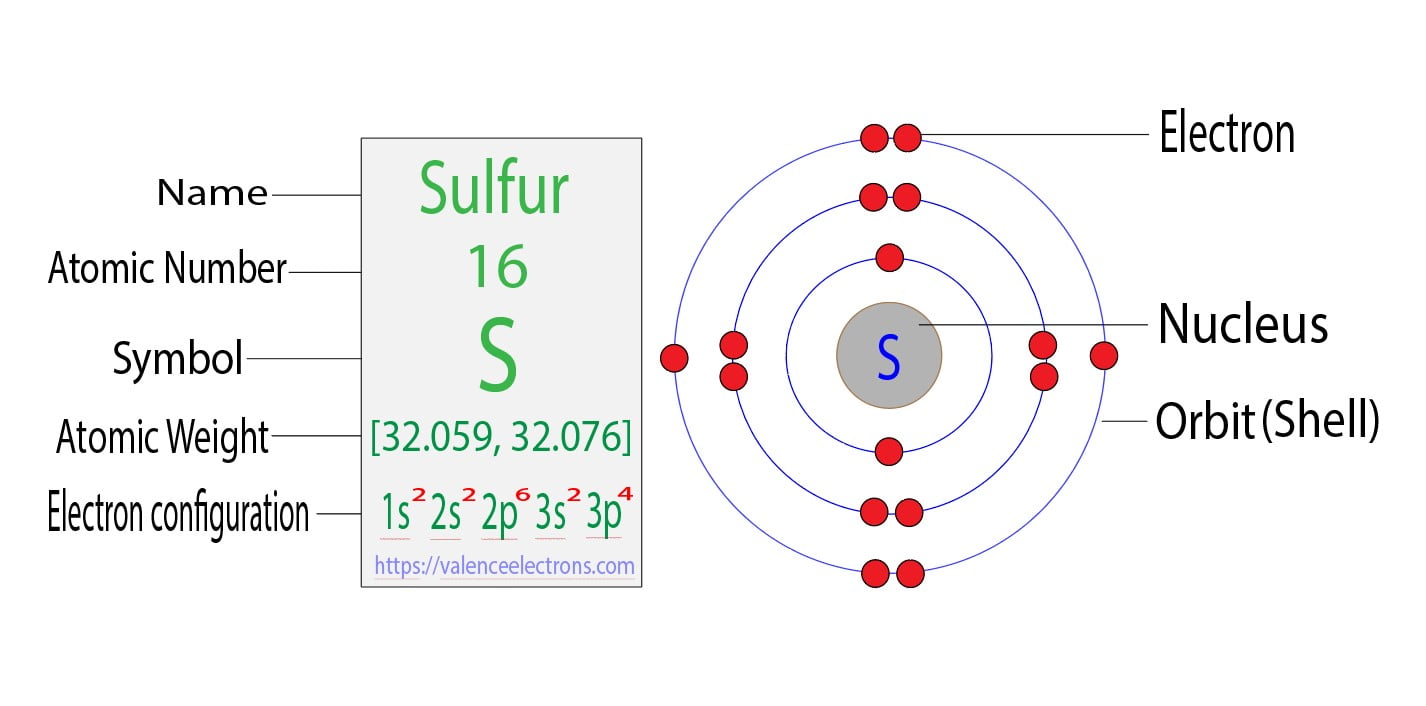
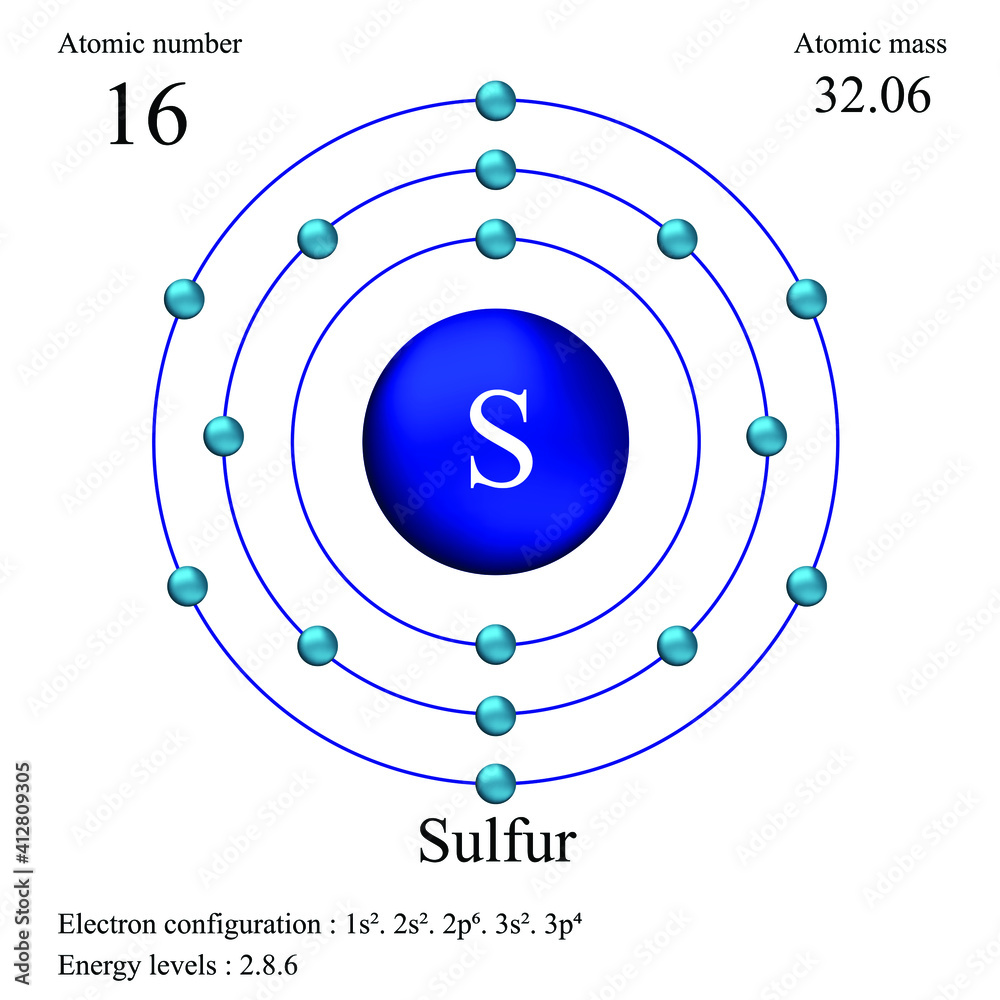
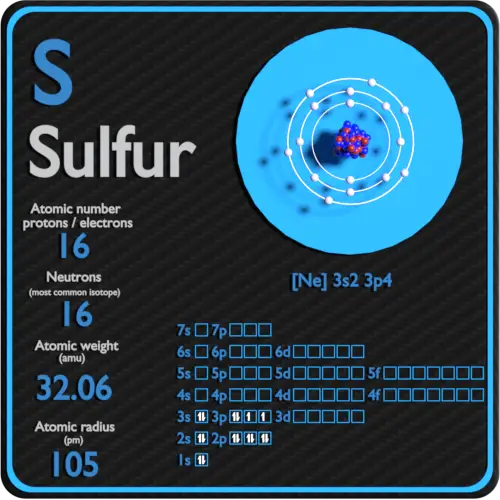
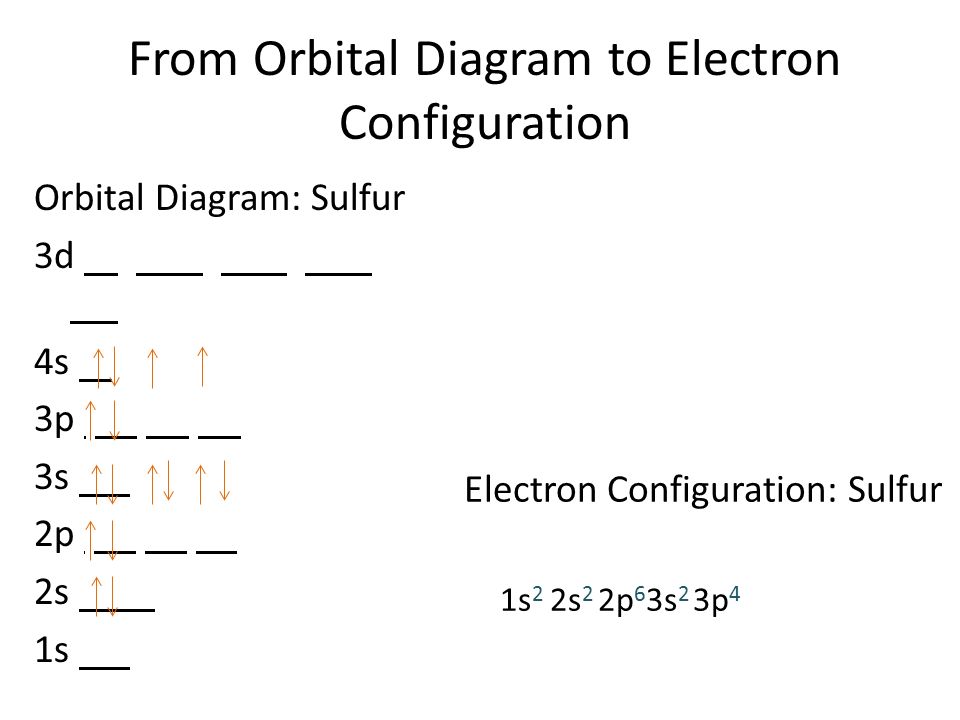
What Is The Valence Electron Configuration For The Sulfur Atom - The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. Valence electrons are the outermost. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. How many valence electrons are in an atom of sulfur? Sulfur has six valence electrons. The 3s orbital of s (sulphur) has two electrons. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons).
The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. The 3s orbital of s (sulphur) has two electrons. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. Valence electrons are the outermost. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). How many valence electrons are in an atom of sulfur? Sulfur has six valence electrons.
The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. The 3s orbital of s (sulphur) has two electrons. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. Sulfur has six valence electrons. How many valence electrons are in an atom of sulfur? In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). Valence electrons are the outermost.
Electron Configuration for Phosphorus (P, P3 ion)
How many valence electrons are in an atom of sulfur? Sulfur has six valence electrons. The 3s orbital of s (sulphur) has two electrons. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are.
Draw The Electron Configuration For A Neutral Atom Of Sulfur.
The 3s orbital of s (sulphur) has two electrons. Sulfur has six valence electrons. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). The electron configuration for sulfur is #1s^2 2s^2p^6.
How to Write the Electron Configuration for Sulfur (S)?
The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. How many valence electrons are in an atom of sulfur? In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). Valence electrons are the.
Sulfur Electron Configuration Clipart (2243341) PinClipart
Sulfur has six valence electrons. How many valence electrons are in an atom of sulfur? In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. The electron configuration for sulfur is #1s^2.
Sulfur Electron Configuration Jacks Of Science
The 3s orbital of s (sulphur) has two electrons. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. Valence electrons are the outermost. The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. In order to write the sulfur electron configuration we first need to.
Sulfur Electron Configuration Jacks Of Science
In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). Sulfur has six valence electrons. Valence electrons are the outermost. The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. The electronic configuration of.
How to Find the Valence Electrons for Sulfur (S)?
The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. Valence electrons are the outermost. Sulfur has six valence electrons. How many valence electrons are in an atom of sulfur? The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4.
Sulfur Electron Configuration Clipart Pinclipart Hot Sex Picture
The 3s orbital of s (sulphur) has two electrons. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4. Sulfur has six valence electrons. The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. How many valence electrons are in an atom of sulfur?
Sulfur Protons Neutrons Electrons Electron Configuration
Sulfur has six valence electrons. The 3s orbital of s (sulphur) has two electrons. How many valence electrons are in an atom of sulfur? Valence electrons are the outermost. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4.
Sulfur Electron Configuration (S) with Orbital Diagram
In order to write the sulfur electron configuration we first need to know the number of electrons for the s atom (there are 16 electrons). Valence electrons are the outermost. The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2. Sulfur has six valence electrons. The 3s orbital of.
How Many Valence Electrons Are In An Atom Of Sulfur?
The 3s orbital of s (sulphur) has two electrons. Sulfur has six valence electrons. Valence electrons are the outermost. The electronic configuration of the s (sulphur) atom's valence shell = 3s2 3p4.
In Order To Write The Sulfur Electron Configuration We First Need To Know The Number Of Electrons For The S Atom (There Are 16 Electrons).
The electron configuration for sulfur is #1s^2 2s^2p^6 3s^2 3p^4# this means that sodium has a valence shell of #[ne] 3s^2.