What Species Has The Electron Configuration Ar 3D2
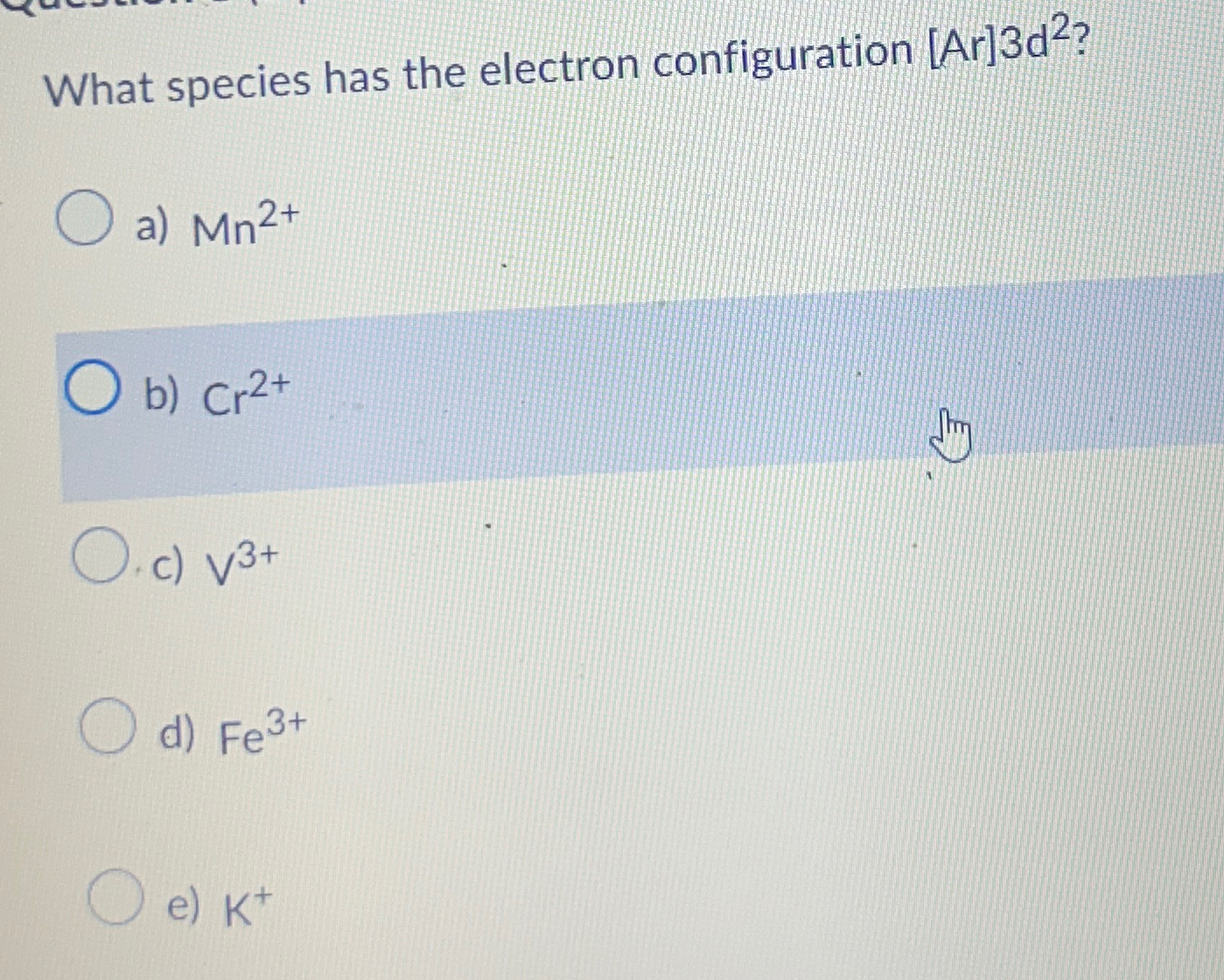
What Species Has The Electron Configuration Ar 3D2 - There are 2 steps to solve this one. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 Argon (a r) has an atomic number of 18. What species has the electron configuration [ar]3d2? In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What species has the electron configuration [ar]3d2? What is the electron configuration for the fe3+ ion? A chemical bond that involves. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two.
What species has the electron configuration [ar]3d2? A chemical bond that involves. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. What species has the electron configuration [ar]3d2? In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What is the electron configuration for the fe3+ ion? What species has the electron configuration [ar]3d2? [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 There are 2 steps to solve this one. Argon (a r) has an atomic number of 18.
There are 2 steps to solve this one. What species has the electron configuration [ar]3d2? The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. What is the electron configuration for the fe3+ ion? A chemical bond that involves. In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. What species has the electron configuration [ar]3d2? Argon (a r) has an atomic number of 18.
Solved What species has the electron configuration
[ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What species has the electron configuration [ar]3d2? The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. What species has the electron configuration [ar]3d2?
Solved What species has the electron configuration
Argon (a r) has an atomic number of 18. What species has the electron configuration [ar]3d2? What species has the electron configuration [ar]3d2? There are 2 steps to solve this one. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two.
What Species Has the Electron Configuration Ar 3d2
[ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10 In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). Argon (a r) has an atomic number of 18. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which.
Electron Configuration of an Atom JavaLab
What species has the electron configuration [ar]3d2? Argon (a r) has an atomic number of 18. What species has the electron configuration [ar]3d2? A chemical bond that involves. What species has the electron configuration [ar]3d2?
What Species Has the Electron Configuration Ar 3d2
There are 2 steps to solve this one. A chemical bond that involves. What species has the electron configuration [ar]3d2? What is the electron configuration for the fe3+ ion? [ar]4s13d6 [ar]4s03d7 [ar]4s03d5 [ar]4s23d9 [ne]3s23p10
Argon Electron Configuration (Ar) with Orbital Diagram
Argon (a r) has an atomic number of 18. [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. What is the electron configuration for the fe3+ ion? A chemical bond that involves.
What Species Has the Electron Configuration Ar 3d2
In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What is the electron configuration for the fe3+ ion? What species has the electron configuration [ar]3d2? What species has the electron configuration [ar]3d2? [ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+.
Argon Electron Configuration (Ar) with Orbital Diagram
What species has the electron configuration [ar]3d2? The electron configuration [ar]3d² indicates an atom that has the same electron configuration as argon (which has 18 electrons) plus two. There are 2 steps to solve this one. In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What species has the electron.
Answered What species has the electron… bartleby
[ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). There are 2 steps to solve this one. Argon (a r) has an atomic number of 18. What species has the electron configuration [ar]3d2?
What Species Has the Electron Configuration Ar 3d2
Argon (a r) has an atomic number of 18. There are 2 steps to solve this one. What species has the electron configuration [ar]3d2? In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). What species has the electron configuration [ar]3d2?
[Ar]4S13D6 [Ar]4S03D7 [Ar]4S03D5 [Ar]4S23D9 [Ne]3S23P10
What species has the electron configuration [ar]3d2? What species has the electron configuration [ar]3d2? What species has the electron configuration [ar]3d2? What is the electron configuration for the fe3+ ion?
The Electron Configuration [Ar]3D² Indicates An Atom That Has The Same Electron Configuration As Argon (Which Has 18 Electrons) Plus Two.
[ne]3s23p6 what species has the electron configuration [ar]3d2?a) mn2+ b) cr2+. In a covalent bond the atoms share the electron pair (s) in order to complete each others octet (valence). A chemical bond that involves. There are 2 steps to solve this one.