What Type Of Reaction Is Caco3 Cao Co2
What Type Of Reaction Is Caco3 Cao Co2 - During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). The type of reaction caco₃ → cao + co₂ is b. Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2). The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e. So, the reaction can be. In a decomposition reaction, a single compound. Complex redox reactions, especially in acidic or basic solutions.
Complex redox reactions, especially in acidic or basic solutions. Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. In a decomposition reaction, a single compound. Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e. The type of reaction caco₃ → cao + co₂ is b. During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). So, the reaction can be. In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2).
Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). Complex redox reactions, especially in acidic or basic solutions. In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2). So, the reaction can be. The type of reaction caco₃ → cao + co₂ is b. The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e. Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. In a decomposition reaction, a single compound.
[ANSWERED] CaCO3 s CaO s CO2 g The standard enthalpy of reaction for
Complex redox reactions, especially in acidic or basic solutions. So, the reaction can be. During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. The given reaction is an example of a decomposition.
Solved Consider the reaction Caco3(S) Cao(s) + CO2(g) When
During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2). Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. The type of reaction caco₃ → cao.
Solved 1. Consider the reaction CaCO3 CaO+CO2 which
Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2). Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. The given reaction is an example of a decomposition reaction as calcium.
Solved Given The Reaction CaCO3 (s) → Cao (s) + CO2 (9) A...
In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2). The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e. Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as.
Figure 1 from Comparative Analysis of CaCO3/CaO Reaction System
The type of reaction caco₃ → cao + co₂ is b. During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. So, the reaction can be. In a decomposition reaction, a single compound.
Solved CaO(s) + CO2(g)CaCO3(s) This reaction is the reverse
Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. So, the reaction can be. The type of reaction caco₃ → cao + co₂ is b. Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao).
[Solved] Consider the reaction [ mathrm{CaCO}_{3}(s)
The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e. In a decomposition reaction, a single compound. During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2). Complex redox reactions, especially in acidic or basic solutions. Caco3 → cao + co2 can be.
SOLUTION A thermodynamics the reaction caco3 cao co2 has a en Studypool
In a decomposition reaction, a single compound. The type of reaction caco₃ → cao + co₂ is b. Complex redox reactions, especially in acidic or basic solutions. So, the reaction can be. The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e.
Solved For the reaction CaCO3( s)→CaO(s)+CO2( g)
Complex redox reactions, especially in acidic or basic solutions. Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. The type of reaction caco₃ → cao + co₂ is b. The given reaction is an example of a decomposition reaction as calcium carbonate decomposes into two new products i.e. During a.
Answered The chemical reaction CaCO3 CaO + CO2… bartleby
Complex redox reactions, especially in acidic or basic solutions. Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. So, the reaction can be. In a decomposition reaction, a single compound. In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2).
In A Decomposition Reaction, A Single Compound.
In the given reaction, calcium carbonate (caco3) decomposes into calcium oxide (cao) and carbon dioxide (co2). Complex redox reactions, especially in acidic or basic solutions. Caco3(s) → cao(s) + co2(g) => it is a decomposition reaction as calcium carbonate undergoes decomposition and the carbon. So, the reaction can be.
The Given Reaction Is An Example Of A Decomposition Reaction As Calcium Carbonate Decomposes Into Two New Products I.e.
Caco3 → cao + co2 can be classified as a decomposition reaction.decomposition reaction. The type of reaction caco₃ → cao + co₂ is b. During a chemical reaction, calcium carbonate (caco3) breaks down to form calcium oxide (cao) and carbon dioxide (co2).
![[ANSWERED] CaCO3 s CaO s CO2 g The standard enthalpy of reaction for](https://media.kunduz.com/media/sug-question-candidate/20220521195736208340-3750862.jpg?h=512)
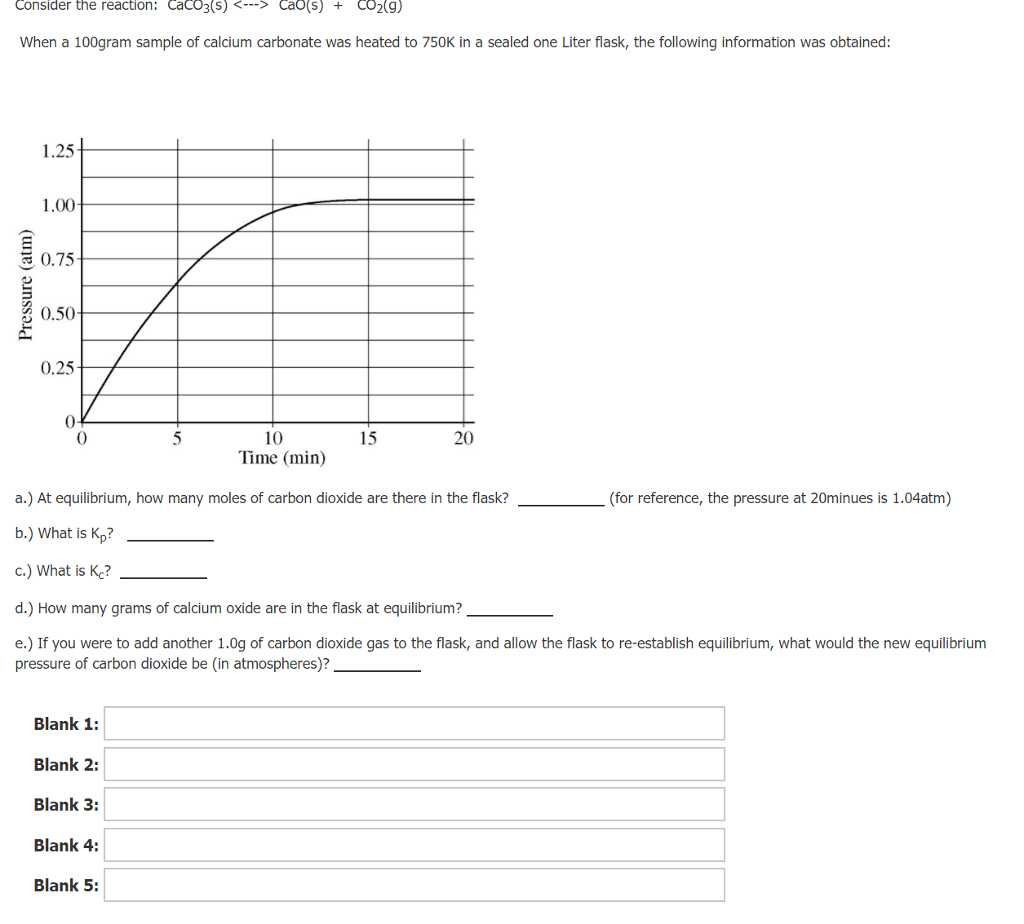
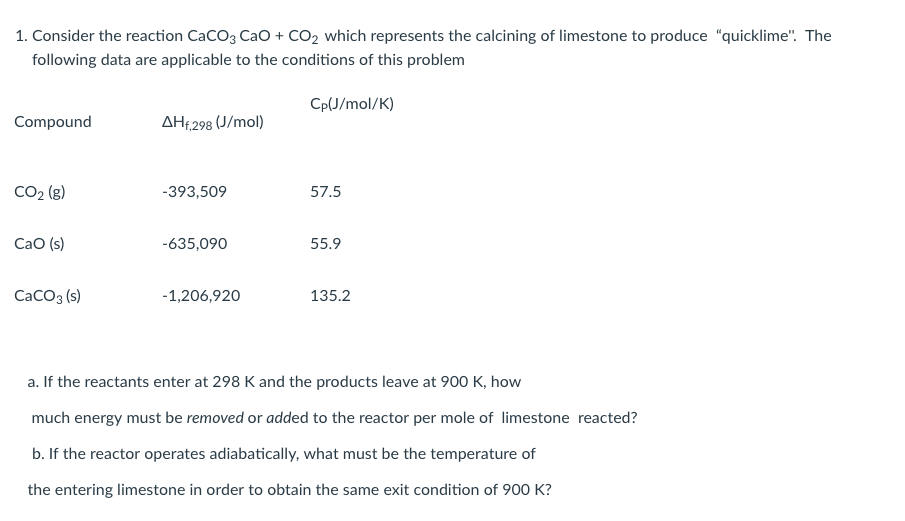
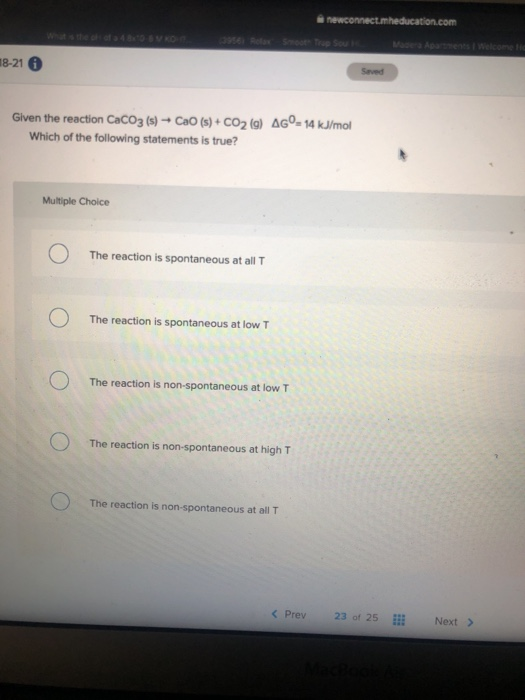

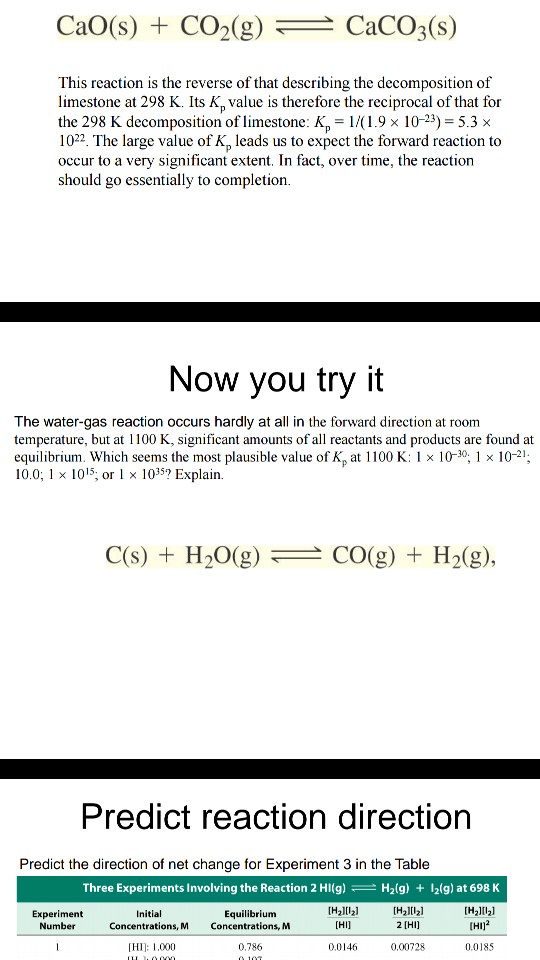
![[Solved] Consider the reaction [ mathrm{CaCO}_{3}(s)](https://media.cheggcdn.com/media/223/2233ae34-9dcc-4076-9c1f-f9e08ed69ec8/phpFhemH2)


