Which Ion Is This Atom Most Likely To Form
Which Ion Is This Atom Most Likely To Form - An atom of selenium(se) forms a monatomic ion. Which atom is most likely to accept electrons to form an ionic bond? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A mercury ion with a negative 2 charge b. 1s2 2s2 2p6 3s1 a. Which ion does it most likely form? A potassium ion with a negative 1.
Which atom is most likely to accept electrons to form an ionic bond? A mercury ion with a negative 2 charge b. An atom of selenium(se) forms a monatomic ion. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: 1s2 2s2 2p6 3s1 a. A potassium ion with a negative 1. Which ion does it most likely form?
Which ion does it most likely form? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A potassium ion with a negative 1. Which atom is most likely to accept electrons to form an ionic bond? 1s2 2s2 2p6 3s1 a. An atom of selenium(se) forms a monatomic ion. A mercury ion with a negative 2 charge b.
Molecular and ionic compounds Chemistry for specialties (2022)
1s2 2s2 2p6 3s1 a. An atom of selenium(se) forms a monatomic ion. Which atom is most likely to accept electrons to form an ionic bond? A mercury ion with a negative 2 charge b. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration:
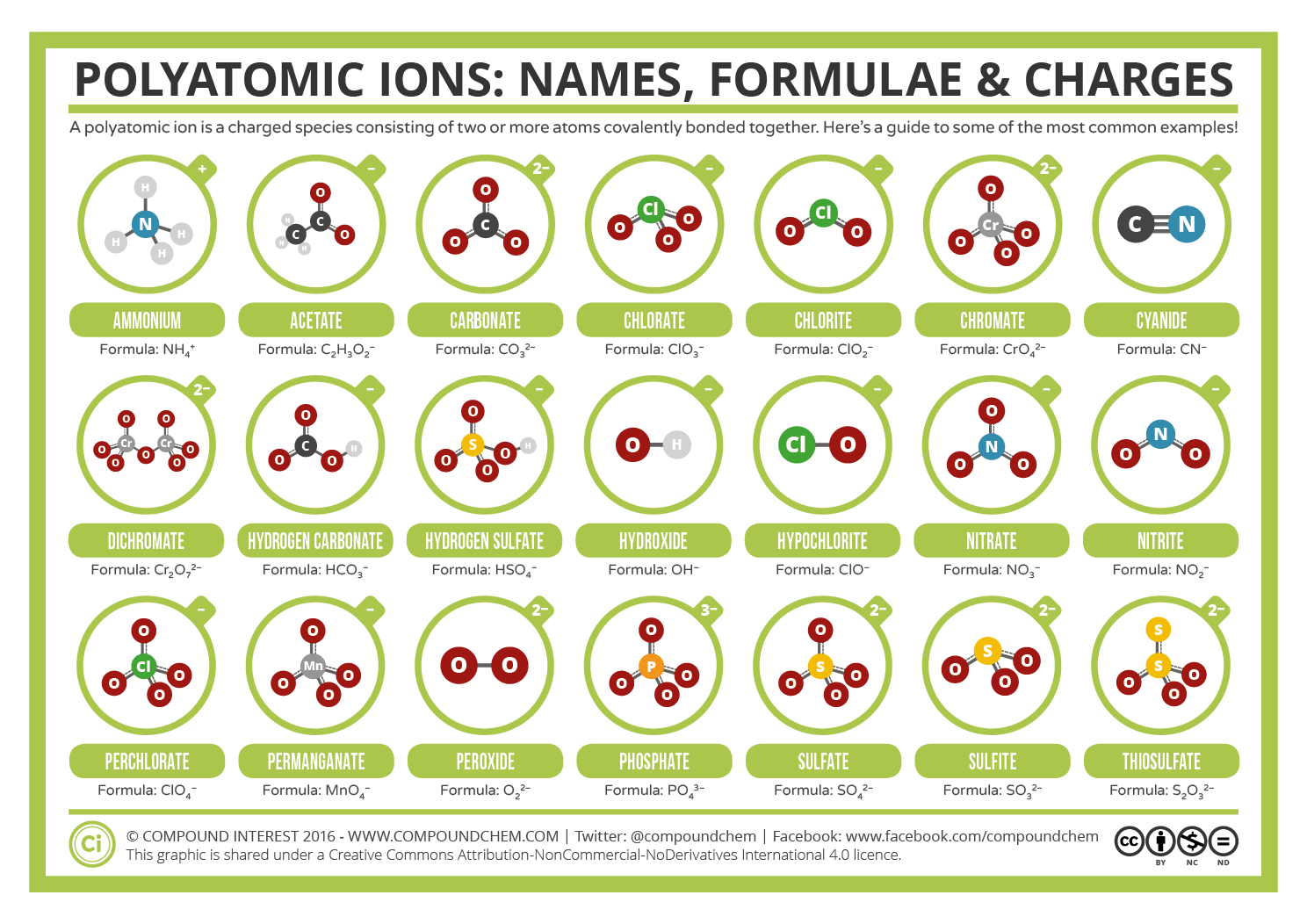
Polyatomic Ions Naming and Formulas Study Guide Inspirit
An atom of selenium(se) forms a monatomic ion. A potassium ion with a negative 1. A mercury ion with a negative 2 charge b. Which atom is most likely to accept electrons to form an ionic bond? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration:
What Are the 3 Major Ions? Infrared for Health
A potassium ion with a negative 1. Which ion does it most likely form? A mercury ion with a negative 2 charge b. An atom of selenium(se) forms a monatomic ion. 1s2 2s2 2p6 3s1 a.
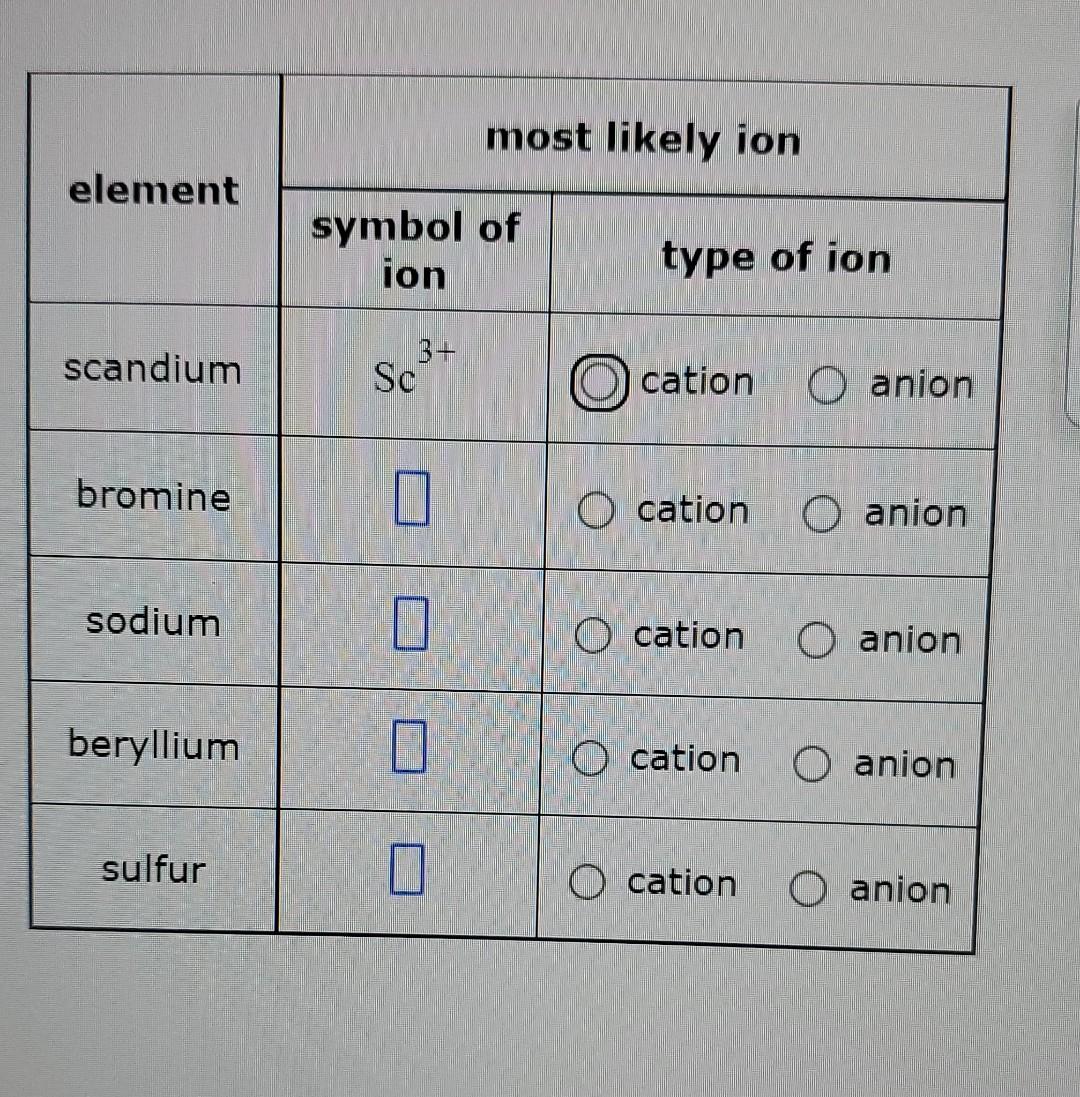
Solved most likely ion element symbol of ion type of ion
A potassium ion with a negative 1. Which ion does it most likely form? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: 1s2 2s2 2p6 3s1 a. An atom of selenium(se) forms a monatomic ion.
Draw the Lewis Dot Structure for the following atoms. Then determine
An atom of selenium(se) forms a monatomic ion. A mercury ion with a negative 2 charge b. A potassium ion with a negative 1. Which atom is most likely to accept electrons to form an ionic bond? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration:
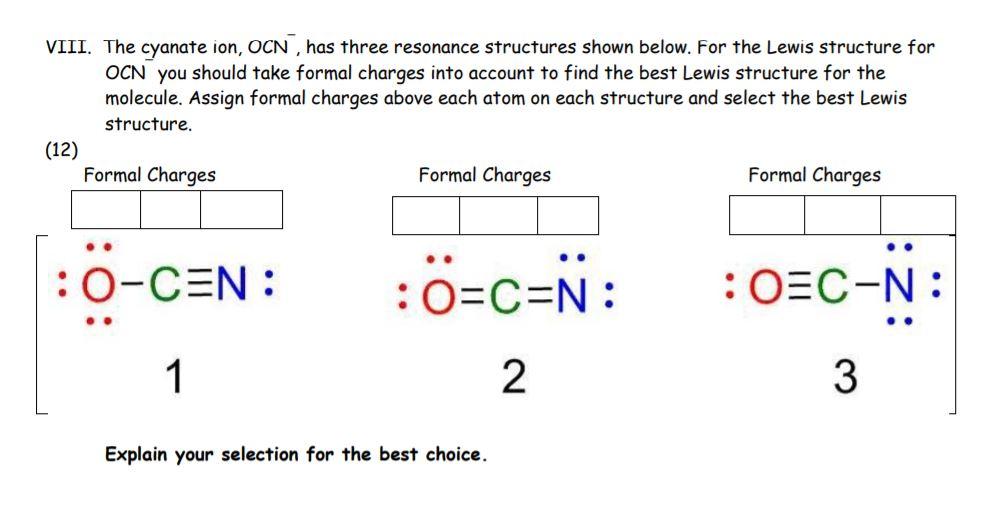
(Solved) VIII. The Cyanate Ion, OCN , Has Three Resonance Structures
Which atom is most likely to accept electrons to form an ionic bond? A potassium ion with a negative 1. Which ion does it most likely form? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: An atom of selenium(se) forms a monatomic ion.
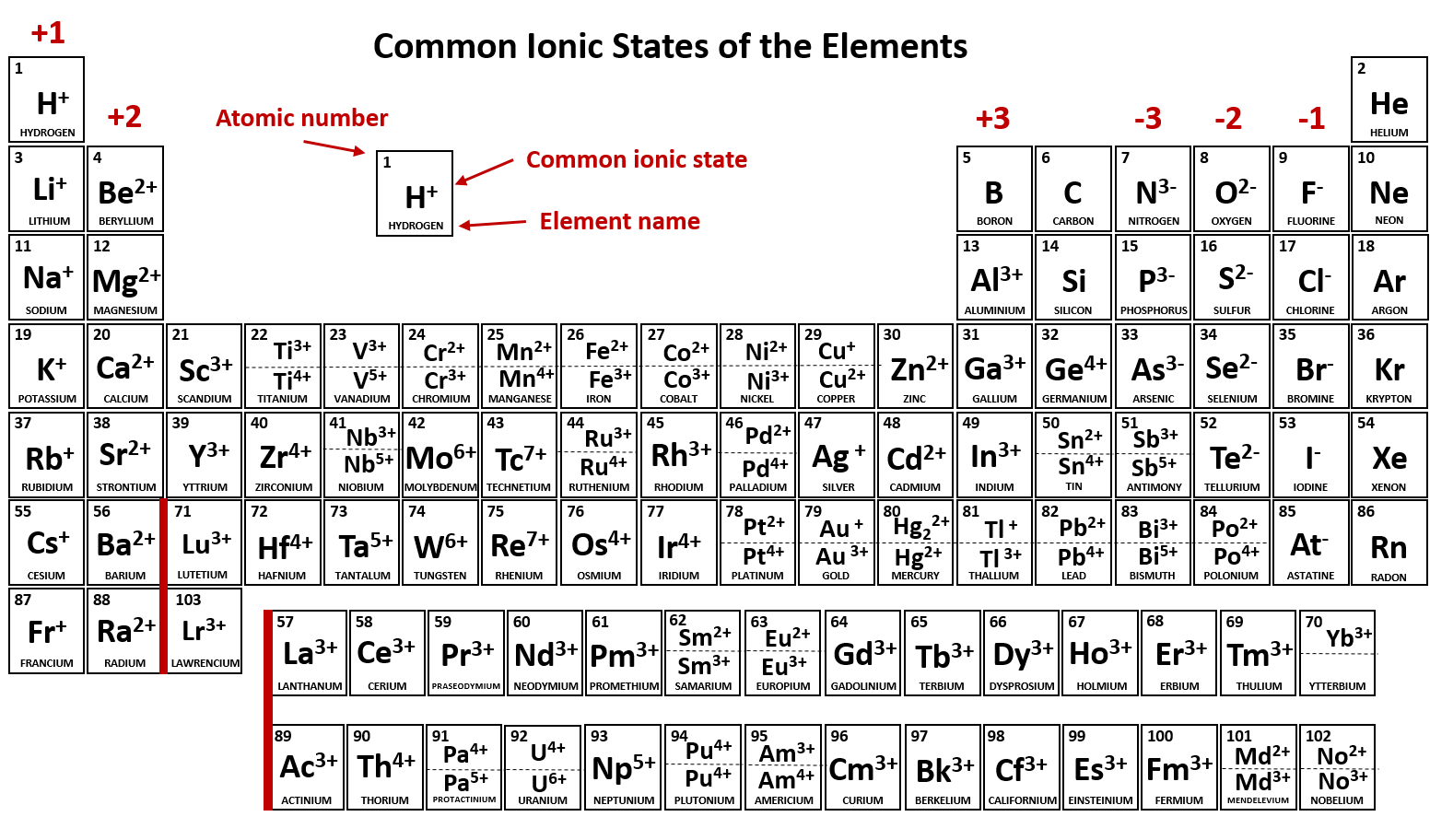
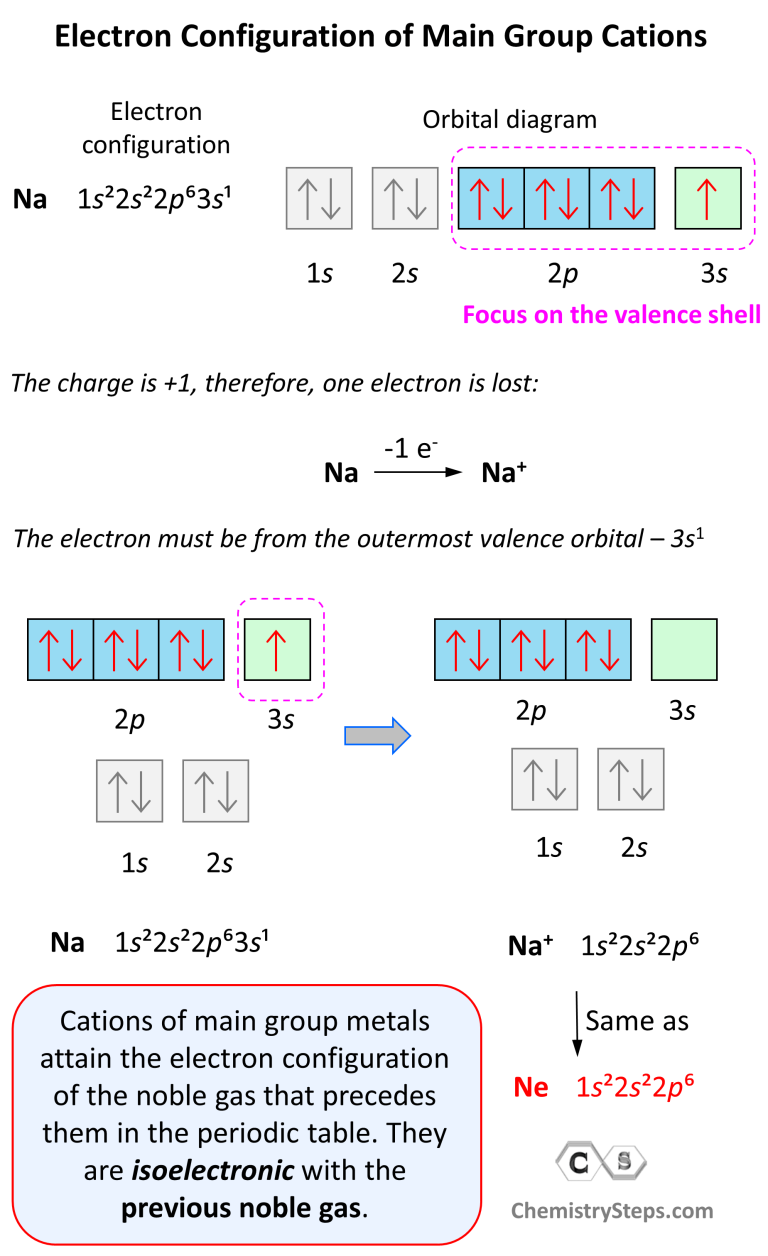
Electron Configurations of Ions Chemistry Steps
Which atom is most likely to accept electrons to form an ionic bond? Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A mercury ion with a negative 2 charge b. A potassium ion with a negative 1. An atom of selenium(se) forms a monatomic ion.
Difference Between Atom And Ion
A mercury ion with a negative 2 charge b. An atom of selenium(se) forms a monatomic ion. Which atom is most likely to accept electrons to form an ionic bond? A potassium ion with a negative 1. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration:
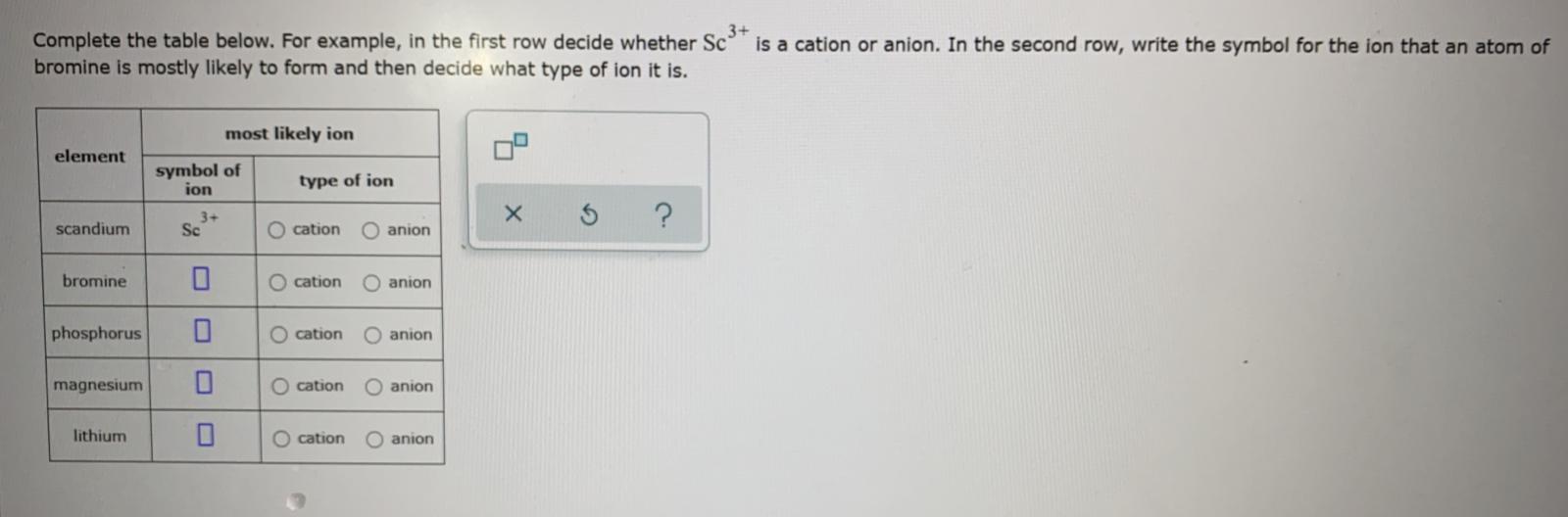
Solved Complete the table below. For example, in the first
Which ion does it most likely form? A mercury ion with a negative 2 charge b. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: Which atom is most likely to accept electrons to form an ionic bond? 1s2 2s2 2p6 3s1 a.
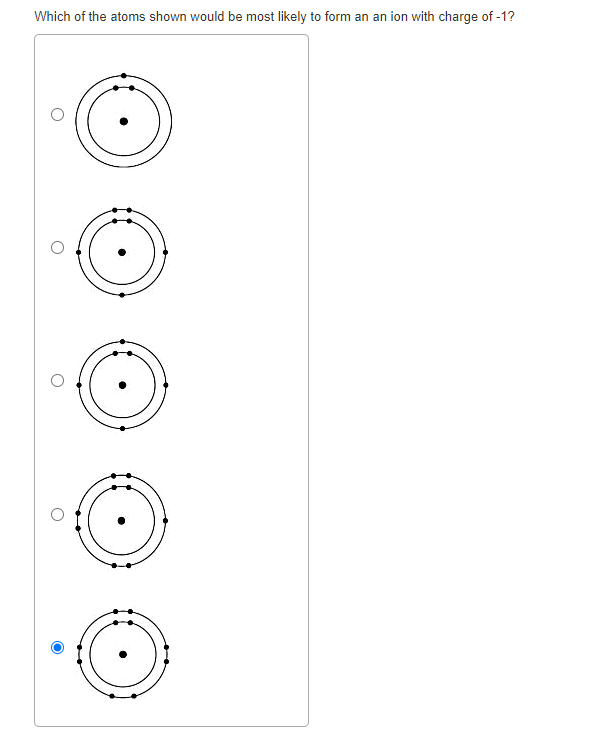
Solved Which of the atoms shown would be most likely to form
1s2 2s2 2p6 3s1 a. A potassium ion with a negative 1. Which atom is most likely to accept electrons to form an ionic bond? A mercury ion with a negative 2 charge b. Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration:
A Mercury Ion With A Negative 2 Charge B.
Study with quizlet and memorize flashcards containing terms like which ion is most likely to form in this configuration: A potassium ion with a negative 1. An atom of selenium(se) forms a monatomic ion. Which atom is most likely to accept electrons to form an ionic bond?
1S2 2S2 2P6 3S1 A.
Which ion does it most likely form?