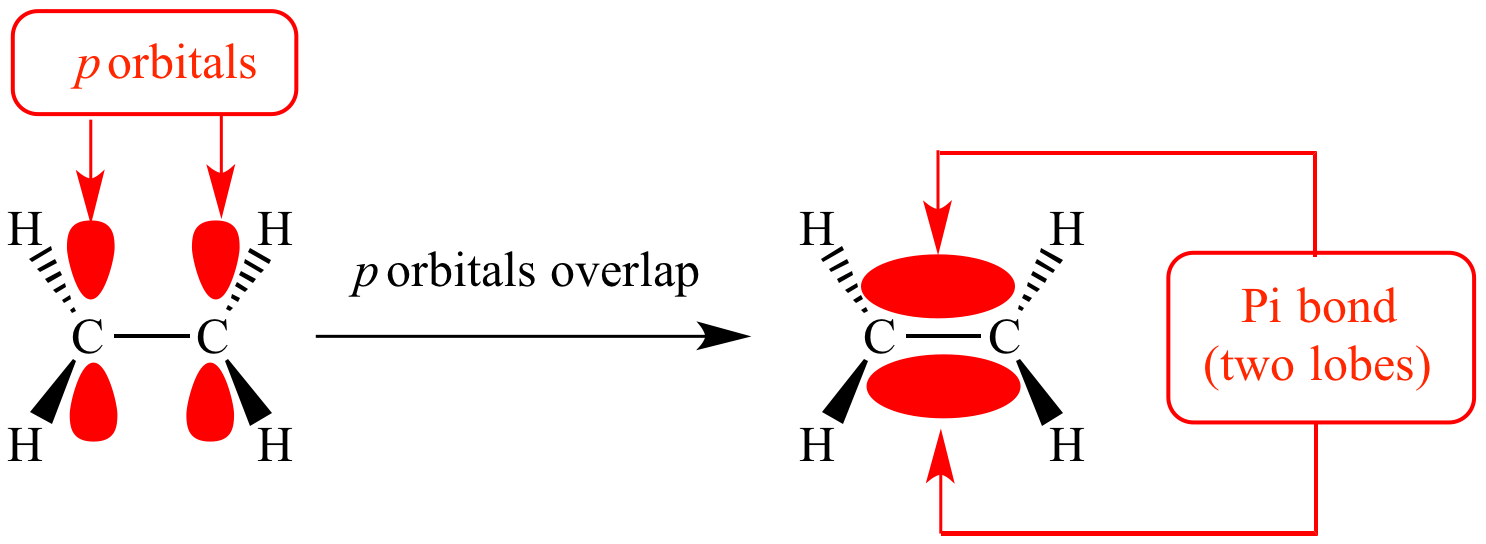
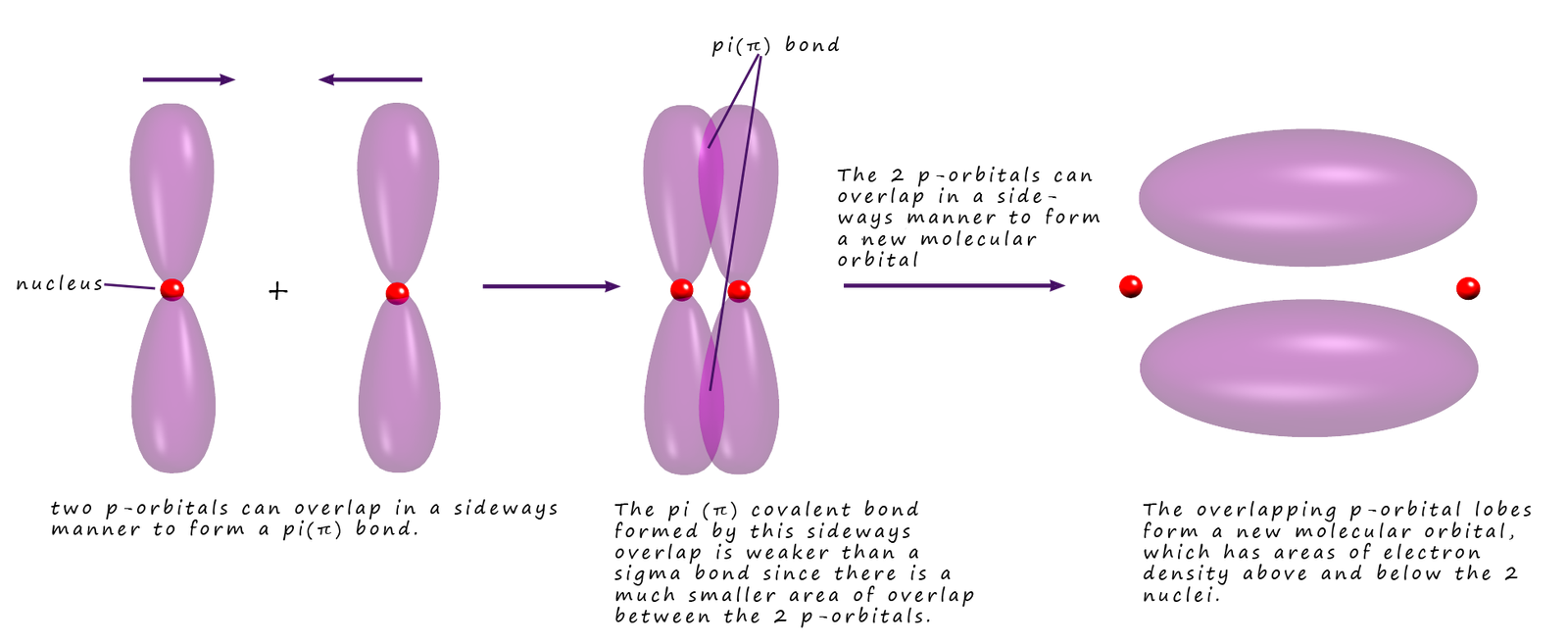
Which Orbitals Form A Pi Bond
Which Orbitals Form A Pi Bond - You can tell what kind of bond forms by how the orbitals. Sigma bonds have no node, pi bonds have one and delta bonds have two. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the.
Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the.
The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals.
Chemistry pi bonds The Student Room
You can tell what kind of bond forms by how the orbitals. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. Sigma bonds have no node, pi bonds have one and delta bonds have two.
Illustrated Glossary of Organic Chemistry sp2 orbital
Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the.
How Do You Define a Pi Bond in Chemistry?
The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. You can tell what kind of bond forms by how the orbitals. Sigma bonds have no node, pi bonds have one and delta bonds have two.
Sigma And Pi Bonds Orbitals
You can tell what kind of bond forms by how the orbitals. Sigma bonds have no node, pi bonds have one and delta bonds have two. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the.
A π bond is formed by the overlap of
The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals.
Orbital Hybridization "Cheat Sheet"? + Example
The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals.
Sigma (δ) and Pi ((π) Bond (ALevel) ChemistryStudent
Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the.
Chapters 9 and 11 study guide
The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. You can tell what kind of bond forms by how the orbitals. Sigma bonds have no node, pi bonds have one and delta bonds have two.
Sigma and pi bonds
Sigma bonds have no node, pi bonds have one and delta bonds have two. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. You can tell what kind of bond forms by how the orbitals.
P Orbitals (Introduction to Pi Bonding) Organic chemistry study, Pi
Sigma bonds have no node, pi bonds have one and delta bonds have two. The in phase overlap forms a pi bonding orbital, which has increased electron density between the nuclei above and below the. You can tell what kind of bond forms by how the orbitals.
The In Phase Overlap Forms A Pi Bonding Orbital, Which Has Increased Electron Density Between The Nuclei Above And Below The.
Sigma bonds have no node, pi bonds have one and delta bonds have two. You can tell what kind of bond forms by how the orbitals.
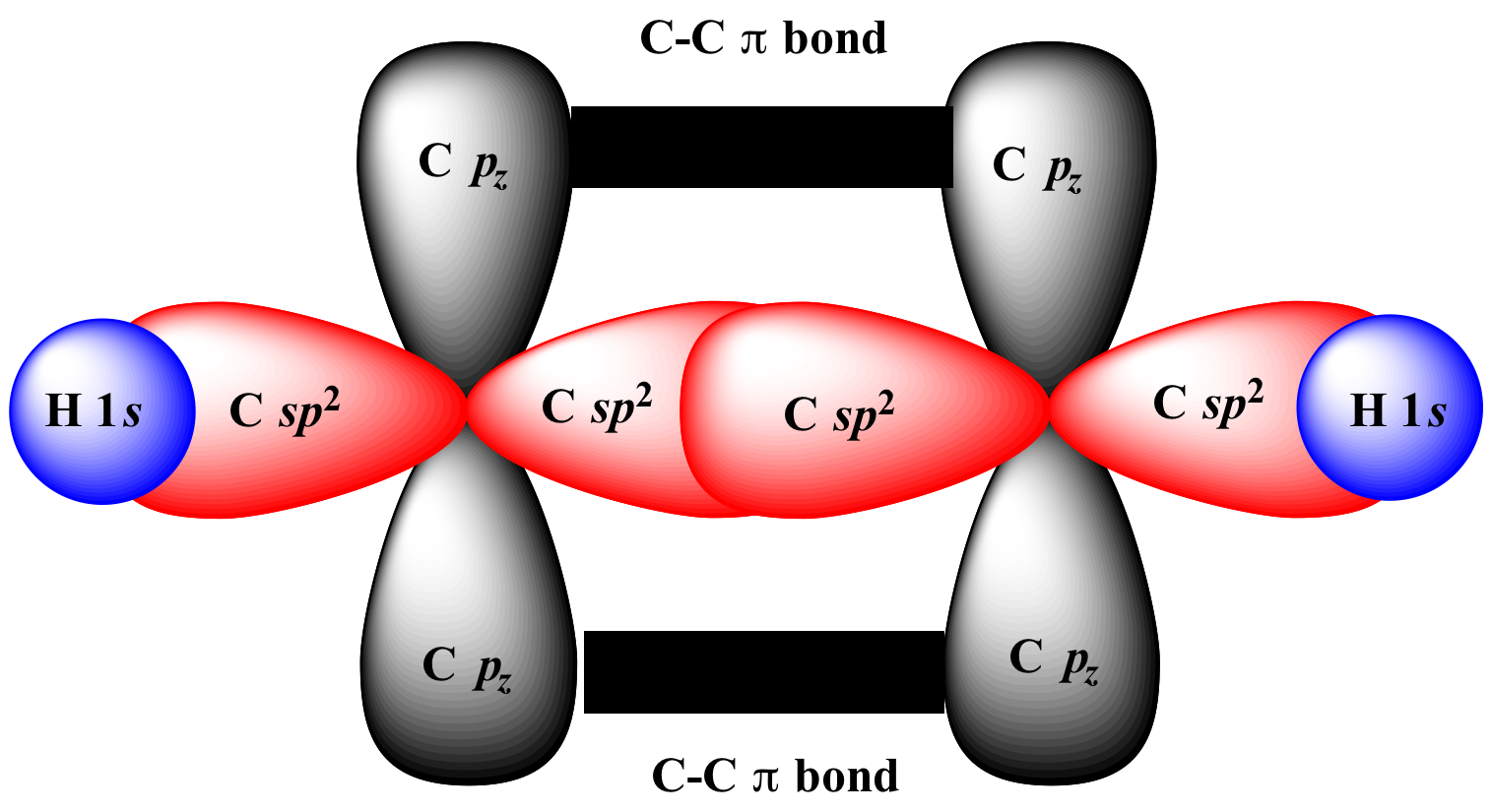
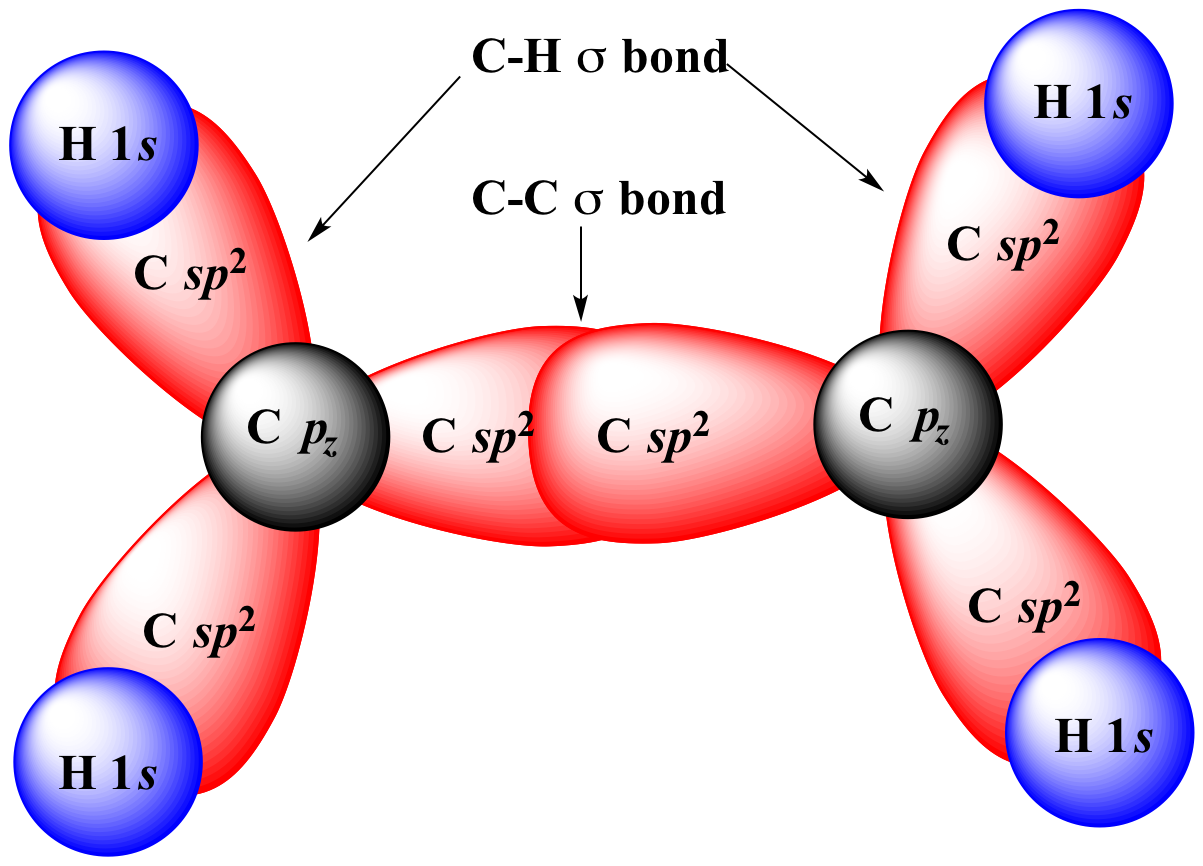
:max_bytes(150000):strip_icc()/1024px-Pi-Bond.svg-d0dece77dabe48c7b13ac7f1ff3d1c4a.jpg)